eISSN: 2378-315X


Research Article Volume 7 Issue 3
1University of Manouba, ISBST, BVBGR-LRES, Biotechpole of Sidi Thabet, Tunisia
2University of Carthage, INRGREF, Unit of Agrosulvopastoralisme, Tunisia
3University of Tunis El Manar, FST, MBA- LR0ES0, Tunisia
Correspondence: Ameur Cherif, LR Biotechnology and Bio?Geo Resources Valorization (LR11ES31), Higher Institute for Biotechnology, University of Manouba Biotechpole of Sidi Thabet, 2020, Sidi Thabet, Ariana, Tunisia
Received: April 18, 2018 | Published: June 11, 2018
Citation: Naili F, Neifar M, Elhidri D, et al. Optimization of the effect of PGPR–based biofertlizer on wheat growth and yield. Biom Biostat Int J. 2018;7(3):226-232. DOI: 10.15406/bbij.2018.07.00213
Halophilic rhizobacteria with potential for alleviation of salt stress in combination with –their plant growth promotion activities would be very useful tools in sustainable agriculture. In the present work, new formulations of biofertilizers have been developed and optimized using Box Bhenken experimental design and response surface methodology. The PGP potential of two halotolerant bacteria Piscibacillus salipiscarius E5 and Halomonas sp. G11, previously isolated from two salt lakes in Tunisian desert (Chott El–Djerid and Sabkhet El–Melah, respectively), was firstly confirmed by their multiple in vitro PGP activities such as phosphate solubilization, nitrogen fixation, indole acetic acid, hydrogen cyanide, siderophore, osmolytes and exopolysaccharides productions. The two selected isolates could potentially act in synergy to support plant growth under salt stress (up to 200mM NaCl). These bacteria were further inoculated with salt–stressed Triticum turgidum durumin (Mahmoudi cv.). Results demonstrated increases in root elongation, root dry weight, shoot elongation and shoot dry weight of inoculated wheat seedlings when compared to uninoculated control plants. The pot culture experiments showed significant response of selected PGP halotolerant bacteria on growth and productivity of durum wheat up to 90 days. The fitted mathematical models allowed us to plot response surfaces as well as isoresponse curves and to determine optimal PGP inoculants under salt stress. Results of this study revealed that using of PGPB biofertilizers especially dual inoculation had significant effects on durum wheat growth and yields.
Keywords: extremely halotolerant PGPB, salt–stressed durum wheat, co–inoculation, statistical optimization, response surface methodology
In their natural environments, plants are constantly subjected to a number of abiotic and biotic stresses that can affect their life and productivity. Saline soils represent about 15% in arid and semi–arid regions and approximately 40% in irrigated lands in the world. High soil salinity adversely affects its physical, chemical and microbiological properties. Also, salt stress results in a significant decrease in productivity of salt–sensitive crops. At present, around 100 countries are affected by salinity induced abiotic stress and this is one of the world’s most serious problem in agriculture.1,2 In Tunisia, salinization, desertification and soil erosion are the most pressing ecological concerns affecting the degradation and loss of productive agricultural lands. About the half of the total irrigated areas are considered at high risk for salinization.3,4 Recently, many projects such as BIODESERT (http://www.biodesert.unimi.it/), MADFORWATER (http://www.madforwater.eu/fr/) and PhosAgro/UNESCO/ IUPAC Green Chemistry for Life (https://www.phosagro.com) were planned and elaborated with the intent of managing microbial resources in arid and saline lands for the development of a sustainable agriculture through improving productivity and quality of vegetable crops and their resistance against biotic and abiotic stresses. Large collections of extremophilic bacteria isolated from arid and saline regions of southern Tunisia were generated and some of them showed a potential to promote plant growth under stressfull conditions.5–9
Plant growth–promoting bacteria (PGPB) are free–living bacteria which promote plant growth and health either directly or indirectly. Direct mechanisms include nitrogen fixation, phosphate solubilization, iron sequestration and phytohormones synthesis.5,6,10,11 Indirect mechanisms include
The identification and characterization of novel salt–tolerant PGPBs has a great interest to promote crop growth and yield in saline soil–based agriculture.1,13–15 Successful application of statistical optimization tools like response surface methodology (RSM) to enhance crop growth and yield has been reported.16,17 However, studies regarding optimization of bioinoculation process under stressfull conditions are still few in the scientific literature. The aim of the present study was to investigate the effects of two novel halophilic PGPB, Piscibacillus salipiscarius E5 and Halomonas sp. G11 on durum wheat growth in the presence and absence of salt stress. To the best of our knowledge, this is the first study to modeling and optimization of salt–stressed durum wheat inoculation with these two bacterial strains.
Bacterial strains and their characterization
Extremely halotolerant strains Halomonas sp. G11 and Piscibacillus salipiscarius E5 used in this study were isolated from Chott el Djerid and Sabkhet el Melah salt–lakes of Southern Tunisia, respectively7. The optimal growths were found at pH 8 in nutrient medium supplemented with 10% w/v NaCl concentration.
Bioassays for plant growth promoting by selected PGPB strains
G11 and E5 strains were screened for their PGP activities such as N2–fixation, inorganic tricalcium phosphate solubilization, production of siderophore, indol acetic acid and ammonia as described by Jensen,18 Nautiyal,19 Alexander and Zuberer,20 Salkowaski’s,21 Cappuccino and Sherman22 and Vijayabaskar et al.23 respectively. All these experiments were conducted at 30°C, pH 8 with addition of 10% NaCl.
To prepare bacterial inocula of desired population size (108 CFU ml−1), G11 and E5 strains were grown in nutrient broth medium supplemented with 10% (w/v) NaCl and incubated at 30°C for 72h. The durum wheat, variety Mahmoudi, with and without bacterial inoculum, was tested for salt tolerance at germination, seedling emergence and early seedling growth in NaCl solutions of different osmotic potentials. Seedling growth was estimated by measuring fresh and dry weights of the different parts of seedlings after drying the samples for 48 h at 80°C.12,24
Experimental design and greenhouse conditions
Three variables BBD combined to response surface methodology25 was carried out to optimize wheat growth promotion by selected halophilic PGPB strains under salt stress pot–experiments. Four seeds were planted in each pot and three replicates were used for each BBD experiment. Each pot was irrigated daily via a drip irrigation system. The greenhouse was maintained at high humidity with a constant temperature of 30±2°C.
The relationship between the wheat grain yield responses (Y1: ear length, Y2: number of spikelets/ear, Y3: number of grains/ear) and the three studied variables, namely salt stress (X1), E5 inoculum size (X2) and G11 inoculum size (X3), was approximated by the following second order polynomial function:
Y = b0 + b1X1 + b2X2 + b3X3 + b11X12 1+ b22 X22 + b33 X32 + b12 X1X2 + b13 X1X3 + b23 X2X3;
Where: Y represent the measured responses; b0, bj, bjk and bjj are the BBD model coefficients. Generation and treatment of BBD data were performed using the specialized experimental design software NemrodW.26
Plant growth promoting potential under saline conditions
salipiscarius E5 and Halomonas sp. G11, previously isolated from two Tunisian salt lakes, showed multiple plant growth promoting traits under saline condition (10% NaCl) such as phosphate solubilization, nitrogen fixation, indole acetic acid, hydrogen cyanide, siderophore, osmolytes and exopolysaccharides productions (Figure 1).
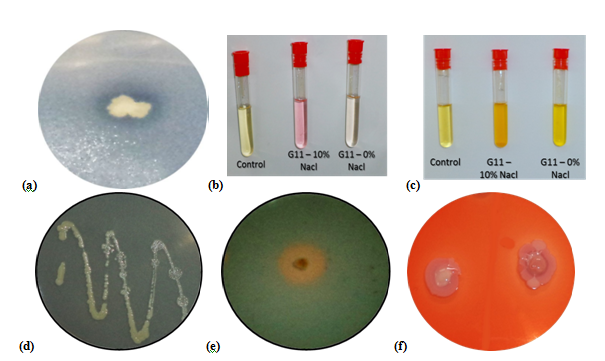
These bacteria were further inoculated with Triticum turgidum durumin (mahmoudi cv.) under salt stress (100 mM NaCl). Results demonstrated increases in root elongation, root dry weight, shoot elongation and shoot dry weight of inoculated wheat plants when compared to uninoculated (control) ones. The two selected PGPB strains can potentially act in synergy to support durum wheat growth under salt stress (Figure 2).
Statistical optimization of bacterial inoculant formula

A BBD was carried out to evaluate the effects of wheat inoculation with P. salipiscarius E5 (0–108CFU/g) and Halomonas sp. G11 (0–108CFU/g) under salt stress (0–200mM) on the grain yields (Ear length, number of spikelets/ear and number of grains/ear). The BBD design matrix and the observed and predicted responses were given in Table 1. The model coefficients were determined using the last square method25 and the predicted responses were calculated by NemrodW software.26
Experience N° |
Salt stress |
E5 inoculum |
G11 inoculum |
Experimental ear length (cm) |
Theoretical ear length (cm) |
Experimental number of spikelets/ear |
Theoretical number of spikelets/ear |
Theoretical number of spikelets/ear |
Theoretical number of grains/ear |
1 |
0 |
0 |
0.5 |
4.25 |
4.047 |
8.5 |
8.063 |
14.5 |
13.625 |
2 |
200 |
0 |
0.5 |
3.125 |
3.203 |
6.25 |
6.25 |
8 |
8.063 |
3 |
0 |
1 |
0.5 |
4 |
3.922 |
7.75 |
7.75 |
12.25 |
12.188 |
4 |
200 |
1 |
0.5 |
3.25 |
3.453 |
6 |
6.438 |
8.5 |
9.375 |
5 |
0 |
0.5 |
0 |
3.875 |
4.125 |
7.5 |
7.906 |
10.75 |
11.781 |
6 |
200 |
0.5 |
0 |
3.375 |
3.344 |
5.75 |
5.719 |
6 |
6.094 |
7 |
0 |
0.5 |
1 |
3.75 |
3.781 |
6.75 |
6.781 |
10.25 |
10.156 |
8 |
200 |
0.5 |
1 |
3.5 |
3.25 |
6.25 |
5.844 |
8.5 |
7.469 |
9 |
100 |
0 |
0 |
3.125 |
3.078 |
6 |
6.031 |
6.75 |
6.594 |
10 |
100 |
1 |
0 |
3.5 |
3.328 |
6.5 |
6.094 |
8.25 |
7.281 |
11 |
100 |
0 |
1 |
2.875 |
3.047 |
5.25 |
5.656 |
6.25 |
7.219 |
12 |
100 |
1 |
1 |
2.875 |
2.922 |
5.5 |
5.469 |
6.25 |
6.406 |
13 |
100 |
0.5 |
0.5 |
3 |
2.75 |
6.25 |
5.938 |
6 |
5.5 |
14 |
100 |
0.5 |
0.5 |
2.5 |
2.75 |
5.75 |
5.938 |
5 |
5.5 |
15 |
100 |
0.5 |
0.5 |
3 |
2.75 |
5.75 |
5.938 |
5 |
5.5 |
16 |
100 |
0.5 |
0.5 |
2.5 |
2.75 |
6 |
5.938 |
6 |
5.5 |
Table 1 Experimental conditions of the BBD in coded and natural variables and the corresponding experimental and theoretical responses (predicted on the basis of second-order regression equations)
The following second order polynomial equations were modeled to determine the optimum requirement of E5 and G11 inoculant concentrations (X1 and X2) and salt concentration (X3) for maximum wheat yields:
Y1 : ear length (cm) = 2.750 – 0.328 X1 + 0.031 X2 – 0.109 X3 + 0.719 X12 + 0.188 X22 + 0.156 X32 + 0.094 X1X2 + 0.063 X1X –0.094 X2X3 (Eq. 1)
Y2: number of spikelets/ear = 5.938 – 0.781 X1 –0.031 X2 – 0.250 X3 + 0.969 X12 + 0.219 X22 –0.344 X32 + 0.125 X1X2+0.313 X1X3–0.063 X2X3 (Eq. 2)
Y3: number of grains/ear = 5.500 – 2.094 X1 –0.031 X2 – 0.062 X3 + 3.656 X12 + 1.656 X22 –0.281 X32 + 0.688 X1X2 + 0.750 X1X3–0.375 X2X3 (Eq. 3);
Where, Y1, Y2 and Y3 were predicted grain yield responses and X1, X2 and X3 were the coded values of NaCl concentration, P. salipiscarius E5 inoculum concentration and Halomonas sp. G11 inoculum concentration, respectively.
The statistical significance and the adequacy of the second– order model equations was checked using analysis of variance (ANOVA). ANOVA of the three developed regression models demonstrated high significance (P<0.001) of the models and an insignificant lack of fit (Table 2), indicating that most of the variability in the responses could be explained by the second–order model equations.
Source of variation |
Sum of squares |
Degrees of freedom |
Mean square |
Ratio |
Significance |
Y1 : ear length (cm) |
|||||
Regression |
104.5742 |
9 |
11.6194 |
10.5481 |
** |
Residuals |
6.6094 |
6 |
1.1016 |
||
Validity |
5.6094 |
3 |
1.8698 |
5.6094 |
9.6% (NS) |
Error |
1 |
3 |
0.3333 |
||
Total |
111.1836 |
15 |
|||
Y2 : number of spikelets/ear |
|||||
Regression |
92.4319 |
9 |
10.2702 |
36.1283 |
*** |
Residuals |
1.7056 |
6 |
0.2843 |
||
Validity |
1.4981 |
3 |
0.4994 |
7.2199 |
7.0% (NS) |
Error |
0.2075 |
3 |
0.0692 |
||
Total |
94.1375 |
15 |
|||
Y3: number of grains/ear |
|||||
Regression |
244.3869 |
9 |
27.1541 |
21.0565 |
** |
Residuals |
7.7375 |
6 |
1.2896 |
||
Validity |
1.6575 |
3 |
0.5525 |
0.2726 |
84.3% (NS) |
Error |
6.08 |
3 |
2.0267 |
||
Total |
252.1244 |
15 |
|||
Table 2 ANOVA for the response surface quadratic BBD models
NS no significant **Significant at the level of 99.0 % ***Significant at the level of 99.9 %
The NemrodW software was used to produce three–dimensional (3D) response surface plots. The 3D surfaces are graphical representations of the regression equation for the optimization of wheat inoculation conditions in the studied experimental domain. In such plots, the response functions were presented as function of two factors while the third one was fixed at a constant level (Figure 3). The response plot revealed that an increase in salt concentration decreased the grain yields. However, the responses increased with the increase of inoculant size.
The optimum operating co–inoculation conditions of salt–stressed durum wheat, carried out numerically by using NemrodW software, were summarized in Table 3.

Salt stress (X1) |
Inoculum E5 (X2) (CFU/g) |
Responses (Y1-2-3) |
50 mM |
E5 = 0.9 108 |
ear length Y1 = 3.38 cm |
G11 = 0.2 108 |
number of spikelets/ear Y2 = 6.76 |
|
number of grains/ear Y3 = 8.55 |
||
100 mM |
E5 = 1.0 108 |
ear length Y1 = 3.25 cm |
G11 = 0.1 108 |
number of spikelets/ear Y2 = 6.14 |
|
number of grains/ear Y3 = 7.27 |
||
200 mM |
E5 = 1.0 108 |
ear length Y1 = 3.42 cm |
G11 = 0.6 108 |
number of spikelets/ear Y2 = 6.40 |
|
number of grains/ear Y3 = 9.37 |
Table 3 Optimal levels of halophilic bacterial inoculants leading to the highest grain yields of durum wheat when grown under salt stress
Figure 4 illustrated the positive effects of bacterial inoculation on the growth of the salt–stressed durum wheat plants.
The goal of this study was to characterize the response of salt–stressed durum wheat to the inoculation by the halophilic PGPB strains, Piscibacillus salipiscarius E5 and Halomonas sp. G11; and to determine if these PGPB could improve durum wheat salt–stress tolerance. In fact, the soluble salts when accumulated in the soil material leads to an increase in soil osmotic pressure, a decrease of plant water absorption and a nutritional imbalance in the plant rhizosphere.27 In the last decade, extensive research has been conducted to unravel the beneficial effects of halophilic PGPB on plant growth.27–31 Siddike et al.28 isolated 140 halotolerant bacterial strains from the soil of barren fields and the rhizosphere of halophytic plants in the vicinity of the Yellow Sea, in the Republic of Korea, exhibiting nitrogen fixation, phosphorus and zinc solubilization, thiosulfate oxidation, production of indole acetic acid, ammonia and extracellular hydrolytic enzymes (protease, chitinase, pectinase, cellulase, and lipase). The inoculation of the halotolerant bacterial strains such as Brevibacterium epidermidis RS15, Micrococcus yunnanensis RS222, and Bacillus aryabhattai RS341 to enhance salt stress (150mM NaCl) in canola plants produced a significant increase in root length and dry weight in comparison with the uninoculated positive controls. Mapelli et al.29 isolated 20 Halomonas strains from Sebkhet and Chott hypersaline ecosystems in Tunisia, having resistance to salinity and drought stresses and possessing PGP activities at 5% NaCl, such as nitrogen fixation, phosphate solubilisation, indol acetic acid and ammonia production. Furthermore, the PGPB Halomonas elongata revealed ability to massively adhere on Salicornia roots, investigating the suitability of halophilic PGPB to set up effective inocula.28 Dasele et al.30 described the plant growth promoting activities of Halobacillus sp. and Halomonas sp. including the production of indole acetic acid, hydrogen cyanide, ammonia, amylase and protease in presence of salinity (21–29% NaCl) and heavy metals (1 mM cobalt, cadmium, and nickel and 0.04 mM mercury and 0.03 mM silver). Rajput et al.31 reported that the halophilic Planococcus rifietoensis strain, presented different plant growth promoting activities (indol acetic acid, phosphatases, ACC deaminase, etc.) that enhance the growth and yield of T. aestivum under salt stress. Similarly, the results of present study revealed that inoculation/co–inoculation with halophilic P. salipiscarius E5 and Halomonas sp. G11 had potential to improve the wheat growth. The plant growth promotion could be the result of the beneficial functions of applied PGPR isolates, like plant growth hormone production, nitrogen fixation, and P solubilization as reported in other studies.24,27–31 Moreover, E5 and G11 strains were able to decrease the significant inhibitory effect of salinity probably via the production of organic osmolytes.
The plant growth promotion effects of P. salipiscarius E5 and Halomonas sp. G11 on the salt–stressed durum wheat were evaluated and validated, experimentally using a BBD and RSM. Based on this experimental design, the durum wheat yields (Ear length, number of spikelets/ear and number of grains/ear) under each set of conditions were determined and compared with the corresponding predicted levels suggested by Nemrod W software. The obtained results suggested that the postulated models can be effectively used to predict the responses in the explored domain. The ANOVA indicated significant effects of salinity stress levels (0–300mM) and PGPB inoculants (0–108CFU/g of soil) on durum wheat yields. Generally, BBD data revealed that salt stress had a dramatic negative effect on the plant responses. The optimum doses of P. salipiscarius E5 and Halomonas sp. G11 for maximum durum wheat yields were found to be dependant to salt stress levels and plant growth stages. These results are consistent with previous reports evaluating the PGP potential of halophilic and halotolerant PGPB strains in salt affected soils.28–31
In conclusion, the use of halophilic PGPB in the agriculture can be considered as interesting and efficient strategy for enhancing crop growth under saline soil. In this study, our results showed that the two halophilic strains Piscibacillus salipiscarius E5 and Halomonas sp. G11, respectively isolated from Chott El–Djerid and Sabkhet El–Melah salt lakes in Tunisian desert, have the potential to improve durum wheat growth under salt stress due to their plant growth promoting properties such as nitrogen fixation, phosphate solubilization and production of stimulatory metabolites (exopolysaccharides, osmolytes, phytohormones, etc.). This study proved also that Box–Behnken experimental design and response surface methodology could be effectively used to modeling and optimizing the co–inoculation of salt–stressed durum wheat by the two halophilic PGPB under pot house conditions, in order to enhance the wheat grain yields, including the ear length, the number of spikelets/ear and the number of grains/ear. The overall results suggest that inoculation/co–inoculation with PGPB could be an effective approach to induce salinity tolerance and improve growth and yield of durum wheat under salt affected conditions compared to the uninoculated plants. Taking into account the search for more conservative and biological agricultural systems, crop inoculation with halophilic PGPB seems to be a promising tool leading to increased agricultural sustainability. Further microbial and physiological studies are required under field conditions and the mechanisms of osmoadaptation and plant growth promotion should be studied at molecular level.
The authors acknowledge financial support from the PhosAgro/UNESCO/IUPAC partnership in the ambit of the Green Chemistry for Life grant programme (project N° 45003552865) and the Tunisian Ministry of Higher Education and Scientific Research in the ambit of the laboratory project LR11ES31.
Author declares that there are no conflicts of interest.

©2018 Naili, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
2 7