Advances in
eISSN: 2572-8490


Review Article Volume 2 Issue 1
Department of Zoology, DDU Gorakhpur University, India
Correspondence: Ravi Kant Upadhyay, Department of Zoology, D D U Gorakhpur University, Gorakhpur, UP, India
Received: December 21, 2016 | Published: February 28, 2017
Citation: Upadhyay RK. Role of biological scaffolds, hydro gels and stem cells in tissue regeneration therapy. Adv Tissue Eng Regen Med Open Access. 2017;2(1):121-135. DOI: 10.15406/atroa.2017.02.00020
Present review article emphasizes role of biological scaffolds, hydrogels and stem cells in tissue engineering mainly in regeneration or repairing of damaged tissues. Highly porous scaffold biomaterials are developed which act as templates for tissue regeneration and potentially guide the growth of new tissue. Present article also describes de-cellularization, cell printing, vascularization, integration of cross linking of methods for scaffolding of biomaterials to reproduce and recapitulate complexity in engineered tissues. It also explains different scaffold types, polymer hydrogels which are necessary for formation of microstructure, cell attachment, differentiation, tissue vascularization and integration. It also explains use of induced pluripotent stem cells (iPSCs) and engineered MSCs as new diagnostic and potential therapeutic tools to remove sustained damage and complications from organ failures. These engineered MSCs assist in making self-assembling supramolecular hydrogels, which have larger applications in cell therapy of intractable diseases and tissue regeneration. No doubt development of more advanced biomaterials, growth factors and stem cell derived products/factors and tissue transplantation methods based on cell regeneration programming will revolutionize the clinical therapeutics. This article suggests identification of new biomaterials/biomolecules such as growth factors, scaffolds, integration, adhesion and regulatory molecules and cell secreted factors which can induce major metabolic and signaling pathways during phase of tissue repairing and induction of regeneration. This innovative research area needs many conceptual improvements in organ therapies, methods and technological advancement to scale more services to the human society.
Keywords: regenerative medicine, biomaterials, scaffolds, tissue engineering, extracellular matrix, bioink
2-D, two dimensional; 3D, three dimensional; ECM, extracellular matrix; GelMA, gelatin methacryloyl; CH1, chitosan hyaluronic acid; DG1, dextran gelatin; GEVAC, gelatin vinyl acetate; PCL, polycaprolactone; HCs, hepatocytes; MEMS, micro electromechanical systems; VEGF, vascular endothelial growth factor; PDGF, platelet derived growth factor; PDMS, polydimethyl siloxane; PCL, polycaprolactone; HGF, hepatocyte growth factor; ASC, adipose derived stem cells
Tissue engineering (TE) is a multidisciplinary field that widely concerned with in vitro engineering of tissues and organs. It is based on principles from the fields of materials science, cell and molecular biology, transplantation, and mechanical engineering to have desirable cells, derivative materials and biochemical factors which can aid and increase the repair and regeneration in deficient and injured tissues.1 Todays, tissue engineering processes are used for development of complex tissues or organs, such as heart, muscle, kidney, liver, and lung. But this emerging biomedical field is facing challenges i.e. shortage of biomaterials, cell sources, vascularization of engineered tissues, and design of drug delivery systems. Two-dimensional (2-D) cell culture platforms are made which are suitable planar surfaces for tissue interfaces. Recently, three dimensional (3-D) tissue models are also developed to enhance relevance and sustainability over 2-D devices.2 The specific improvements in 3D systems are related with proper cellular morphology and signaling over time, resulting in cell overgrowth or changes in viability. With the time technology has progressed successfully and widens clinical applications by developing various three dimensional (3D) cultures for formation of organs i.e. bone, skin, liver, kidney and ear. These tissue engineered organs are used to replace or regenerate damaged organs. It is also used for maintaining or restoring organ functions that may have been lost due to aging, accident or disease. Tissue engineering has broad and diverse impacts on a variety of different applications from tissue regeneration to drug screening.
Tissue engineering also assist in regeneration of damaged tissues by combining cells from the body with highly porous scaffold biomaterials. Scaffold biomaterials act as templates for tissue regeneration and guide the growth of new tissue. These bio-materials will be highly useful for clinical management of organ therapeutics of thousands patients are in need of organ transplants and require outstrip supply. Hence, new methods have been developed for stimulating the body's who own repair mechanisms, inducing healing and regenerating mechanism by stem cell transplantation. By seeing the demand, functional requirement of various materials has been increased manifold. However, for developing new biological scaffolds specific cells are culture with normal somatic cells of organs. By de-cellularization and maintaining long-term in vitro cultures new engineered tissues (adipose tissue, cortical brain tissue, intestine, kidney tissue, bone) have been developed in the laboratory. Present article sketches various types of polymer hydrogels, stem cell types and constructs, with important method of decellularization, cell printing, vascularization, cross linking and integration of cells and biomaterials used for scaffolding. This emerging field of regenerative medicine will significantly cut down the demand of organ transplants by replacing with regenerative tissues developed in vitro cultures. The main purpose of tissue engineering and stem cell transplantation is to regenerate tissues and organs3 or replace damaged or diseased organs and tissues.4 In addition, tissue engineering progressively developed strategies which include use of cells, scaffolds, medical devices and gene therapy. This technology also promises to deliver organs grown from patients' own stem cells. In last decade so many methods and functional biomaterials were developed for scaffolding and are used to repair or regenerate an organ or tissue as well as for drug delivery (Figure 1).
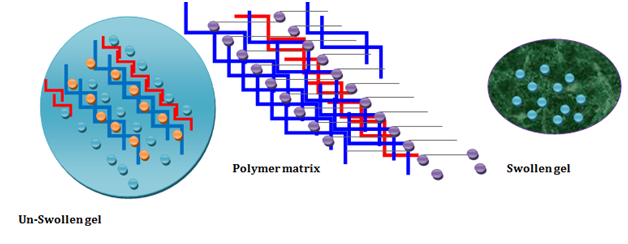
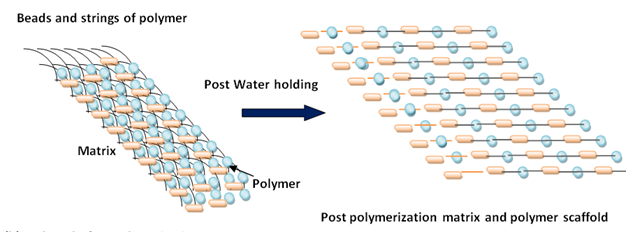

Figure 1 Showing formation of hydrogels and its use in drug delivery.
Biological scaffolds
Scaffolds for tissue engineering are devices that exploit specific and complex physical and biological functions, in vitro or in vivo. Scaffolds are highly specific biomaterials, which are obtained from cell sources, too specific for the engineering of each tissue or organ.1 These are one of the crucial factors for tissue engineering. Scaffolds consisting of natural polymers such as chitosan, gelatin, Tthiol-norbornene. Scaffolds are biocompatible, biodegradable, and bioactive, they have specific pattern for use in tissue engineering for repair and/or regeneration of different tissues including skin, bone, cartilage, nerves, liver, and muscles.5 These biological materials are derived from the extracellular matrix (ECM) of intact mammalian tissues.6 ECMs of different animals and cell sources have been successfully used in a variety of tissue engineering/regenerative medicine and have wide clinical applications.7 (Figure 2). Scaffolds are also produced by using synthetic materials, and their fabrication technologies are derived from already well-established industrial processes. Scaffolds and soluble factors, such as proteins and small molecules are used to induce tissue repair by planting induced somatic cells at the site of injury. These fresh and healthy cells secrete various factors/agents which protect resident fibroblasts and stimulate the migration of these cells into damaged areas, where they proliferate to form new tissue. During regeneration and repairing process biological scaffolds communicate through biochemical and physical signals with cells and with the body micro environment.6
Thiol-norbornene (thiol-ene) photo-click hydrogels have emerged as a diverse material system for tissue engineering applications. These hydrogels are cross-linked through light mediated orthogonal reactions between multi-functional norbornene-modified macromers (e.g., poly (ethylene glycol), hyaluronic acid, gelatin) and sulfhydryl-containing linkers (e.g., dithiothreitol, PEG-dithiol, bis-cysteine peptides) using low concentration of photo initiator. The gelation of thiol-norbornene hydrogels can be initiated by long-wave UV light or visible light without additional co-initiator or co-monomer.8 broad utility of thiol-norbornene hydrogels in tissue engineering and regenerative medicine applications, including valvular and vascular tissue engineering, liver and pancreas-related tissue engineering, neural regeneration, musculoskeletal (bone and cartilage) tissue regeneration, stem cell culture and differentiation, as well as cancer cell biology.8 Similarly, gelatin methacryloyl (GelMA) hydrogels have been widely used for various biomedical applications due to their suitable biological properties and tunable physical characteristics.9 Three dimensional (3D) GelMA hydrogels closely resemble some essential properties of native extracellular matrix (ECM) due to the presence of cell-attaching and matrix metalloproteinase responsive peptide motifs, which allow cells to proliferate and spread in GelMA-based scaffolds. GelMA is also versatile from a processing perspective. It crosslinks when exposed to light irradiation to form hydrogels with tunable mechanical properties which mimic the native ECM of GelMA-based hydrogels in a wide range of applications including engineering of bone, cartilage, cardiac, and vascular tissues, among others. Other applications of GelMA hydrogels, besides tissue engineering, include fundamental single-single cell research, cell signaling, drug and gene delivery, and bio-sensing.9 Fabricated three scaffolds as dextran-gelatin (DG1), chitosan-hyaluronic acid (CH1) and gelatin-vinyl acetate (GEVAC).10
New highly innovative nanofibrous scaffolds have been developed for bone tissue engineering through the integration of nanocomposites and biomimetic scaffolds by using advanced and highly specific technologies.11 These nanoscale macroporous scaffolds provide cells both mechanical support and differentiative cues present in cell's microenvironment (Table 1). Further, advancement in regenerative medicine is made as fabrication of scaffold-free cell sheet-based tissue engineering technology. This cell sheet enables transplanted cells to engraft for a long time and assist in maintaining their viability. This technology is used to treat various diseases and in the clinical setting of cornea, esophagus, heart, periodontal ligament, and cartilage using autologous cells. It is a good technique in which transplanted cell sheets not only replace the injured tissue and compensate for impaired function, but also deliver growth factors and cytokines in a spatiotemporal manner over a prolonged period. It leads to promotion of tissue repair.12 Further, process of integration of cells and sufficient vascularization opened so many possibilities for fabrication of human 3-dimensional vascularized dense and intact tissue grafts for regenerative medicine to parenchymal organs.12 This new technology has increased rate of clinical successes and made curing patients with difficult-to-treat diseases and physically impaired function.
Scaffold Material |
Derivation/Synthesized |
Therapeutic Use |
Toxicity |
Biological Properties |
Limitations |
Natural Scaffolds |
|||||
Alginate |
Anionic polysaccharide derived from Brown Seaweed, also known as algin Macrosystis pyrifera |
Form fatty lobules, It is a linear copolymer with homopolymeric blocks of (1-4)-linked β-D-mannuronate (M) and its C-5 epimer α-L- guluronate (G) residue. Calcium alginate is used in different types of medical products including skin wound dressings to promote healin |
Non toxic, Alginate is both food and skin safe. micro-encapsulation, |
Rapid degradation, waterproofing and fireproofing fabrics, various pharmaceutical preparations, such as Gaviscon |
Limited mechanical strength |
Gelatin |
A mixture of protein and peptides derived from Collagen |
Irreversibly hydrolyzed form of collagen Form hydrogels, microsperes and supports adipose tissue engineering, shells of pharmaceutical capsules |
Non toxic, used as gelling agent in food, pharmaceutical drugs |
Rapid degradation stabilizer, thickener, or texturizer in foods such as yogurt, cream cheese, and margarine |
Limited mechanical strength |
Collagen |
A constituent of ECM, irreversibly hydrolyzed gelatin, Collagen scaffolds are used in tissue regeneration |
Improves ADCC differentiation, ideal for the deposition of cells, such as osteoblasts and fibroblasts, construction of the artificial skin substitutes used in the management of severe burns |
Non toxic, collagen also has many medical uses in treating complications of the bones and skin. |
Rapid degradation, Collagen is used in bone grafting, pore structure, permeability, hydrophilicity and it is stable in vivo |
Limited mechanical strength |
Silk |
Protein polymers obtained from insect larvae, silkworm Bombyx mori reared in captivity |
Surface modification biodegradable biopolymers for bone tissue engineering The fibroin-heavy chain is composed mostly of beta-sheets, due to a 59-mer amino acid repeat sequence with some variations, silk fibroin is recognized as an ideal material for its impressive cytocompatibility, slow biodegradability, and excellent mechanical properties |
Non toxic, its fibres strong and resistant to breaking Silk is a poor conductor of electricity and thus susceptible to static cling. |
Low immunogenicity, decellularized trabecular bone (DCB) included as a "gold standard" pore sizes (250-500 μm vs. 500-1000 μm, HFIP-derived silk fibroin scaffold |
In fibers high mechanical strength. porous silk scaffolds provide a suitable niche to maintain long survival and function of the implanted cells for bone regeneration Its elasticity is moderate to poor, tunable architecture and mechanical properties, porous silk scaffolds |
Synthetic Scaffolds |
|||||
Poly-I-Lactic acid (PLA) or polylactide |
Biodegradable thermoplastic polymer, |
Reproducible fabrication protocols; Easy surface modification; adds mechanical strength maintains adipogenic |
Non toxic |
Rapid degradation is a biodegradable and bioactive thermoplastic aliphatic polyester derived from renewable resources, such as corn starch (in the United States and Canada), tapioca roots, chips or starch (mostly in Asia), or sugarcane |
PLA depolymerase, can also degrade PLA PLDLLA/TCP scaffolds for bone engineering. |
Poly(lactic-co-glycolic acid(PLGA) |
Polyster porous scaffold 75% lactic acid and 25% glycolic acid |
Easily mass produced; Can be modified with addition of motifs/groups or altering topography to improve cell adhsions/proliferation/differenetiation; Promote adipogenic differentiation of ADCC in vitro |
Non toxic, Drug delivery of amoxicillin for the treatment listeriosis |
Biodegradable and biocompatible, requires surface modification to optimize cell growth and differentiation |
Adverse effects of degradation products (inflammation) |
Polycaprolactone(PCL) |
Biodegradable polymer with a low melting point of around 60 °C |
Reproducible ,Fabrication protocols Can be modified with addition of motifs/groupsor altering topography to improve cell adhesion/differentiation/proliferation Adds mechanical strengthPromotes ADSC adipogenesis in vivo |
Non toxic, used as a drug delivery device, PCL beads for controlled release and targeted drug delivery |
Potentially unstable Degradation, degradation which is even slower than that of polylactide clostridium can degrade PCL under anaerobic conditions. |
Used for root canal filling material Very high fixing |
Polyethylene glycol (PEG) |
Polyether compound oligomer or polymer of ethylene oxide. |
Licensed for Medicinal use in laxative and commercially in cosmetic products, Reproducible Fabrication protocols Water soluble and biodegradable enzymatically PEG based gels promote formation of adipose like tissue structures |
Low toxicity, surfactants, in number of laxatives. foods, PEG-coated gene therapy vectors |
Does not provide mechanical strength, Does not provide mechanical strength; requires surface as cell properties as scaffold conjugation ; Rapid degradation, |
Allows a slowed clearance of the carried protein from the blo od, PEG polyols impart flexibility to polyurethanes for applications such as elastomeric fibers |
Biological Scaffold |
|||||
Biological (Decellularized matrix |
Generation through decellularization of tissue to obtain extracellular matrix (ECM, LEM, BEM) |
Widely used in preclinical and clinical tissue engineering studies. Minimal inflammatory responses, allows preservation of structurally organized entities, |
Acts a natural template to guide tissue remodeling and regeneration. native ECM scaffolds in organ regeneration. |
Make minimal inflammatory response, Perfusion decellularization is a novel technology that generates native ECM scaffolds with intact 3D anatomical architecture and vasculature |
Used to from mature adipocyte groups resembling native fat tissue. |
Hybrid/Injectable Scaffolds |
|||||
O-carboxymethyl chitosan (O-CMC) |
Injectable biodegradable Cross-linked hydrogel, O-CMC–SAL PECs have potential biomedical applications due to the nearly neutral complexation pH and the porous structure for drug inclusion |
Reproducible fabrication protocols, can mimic extracellular matrix, uniform distribution when injected into tissues; ADCC demonstrated good adhesion, viability, proliferation and differentiation into adipocytes |
nontoxicity, biodegradability, biocompatibility, antibacterial bioactivity, and excellent pH sensitivity |
Rapid degradation, lack of mechanical strength |
greatly influenced pH and ionic strength of the medium |
Polygcolic acid PGLA/hydrogel |
Biodegradable hydrogel/polymer composite |
Reproducible fabrication protocols, increase mechanical strength whilst mimicking ECM; encapsulating cells in fibrin/chitosan prior to seeding onto PLGA scaffold promotes adipogenic differentiation and maintenance of the adipogenesis |
potential to modify surface properties to provide better interaction with biological materials; |
Rapid degradation, excellent biocompatibility biocompatibility; tailored biodegradation rate biomimetic PLGA substrates able to modulate cell interaction for improved substitution, restoration, or enhancement of bone tissue function. |
poor osteoconductivity and exhibits suboptimal mechanical properties, |
Alginate/polyvinyl alcohol (PVA) with the inclusion of fibrin nanoparticles |
cross linked fibrin incorporated hydrogel scaffolds |
Can be a hydrogel or hollow fibre porous scaffold, Reproducible fabrication protocols; increase mechanical strength whilst mimicking ECM; incorporation of fibrin into biocomposite scaffold improves adipogenic differentiation of ADSCs |
Non toxic, injectable gels have potential in soft tissue regeneration. |
Unstable degradation/uncontrollable degradation, biocompatible and biodegradable hydrogel blend systems |
Limited compressive strength and elastic moduli. |
Table 1 Showing different types of scaffold materials, their derivation, use and biological properties.
After biological scaffold constructs, second important aspect is confined to the surface properties of a biomaterial or a device because it determines the success of the implant in biomedicine, as the majority of biological reactions in human body occur on surfaces or interfaces. For development of an organ from decellularized ECM or formation of cell sheet both cell adhesion and proliferation are highly important processes. These are influenced by micro- and nano-surface characteristics of biomaterials and devices. More specifically, in organ bioengineering cells are seeded on biological or synthetic scaffolding materials ex vivo and allowed to either mature in bioreactors or be implanted without undergoing any maturation.13 As the extracellular matrix is capable of driving complexity during development, hence greater attention must be paid to nanoscale cues to recapitulate complexity in engineered tissues. But mimicking the natural extracellular matrix is one of the critical and challenging technological barriers for scaffold engineering. In addition, for obtaining stem cell derived structures and nanoscale devices mesenchymal stem cells seeded in co-culture can be regulated through physical interaction with specific nanotopographical cues. Therefore, guided stem cell proliferation, differentiation and function are of great importance in the regeneration of 3D tissues and organs using tissue engineering strategies. Nanotopography put impact on mesenchymal stem cells and assists in developing laboratory-based 3D organs and tissues.14 Regenerative medicine offer novel strategies to treat patients with end-stage organ failure. Further, new advancements in tissue technology are waiting to replace aged and genetically defective organs.4
Fibrin biopolymer scaffold
Fibrin is a critical blood component that is extensively used as a biopolymer scaffold in tissue engineering. Fibrin alone or in combination with other materials has been used as a biological scaffold for stem or primary cells to regenerate adipose tissue, bone, cardiac tissue, cartilage, liver, nervous tissue, ocular tissue, skin, tendons, and ligaments.4 Amongst the variety of materials tested, silk fibroin (SF) is increasingly being recognized as a promising material for scaffold fabrication.15 It is easy in processing, show excellent biocompatibility, remarkable mechanical properties and tailorable degradability. It can be used in fabrication of various articles such as films, porous matrices, hydrogels, nonwoven mats, etc. It is also used in various TE applications, including bone, tendon, ligament, cartilage, skin, liver, trachea, nerve, cornea, eardrum, dental, bladder, etc. Commercially available fibrinogen and thrombin are combined to form a fibrin hydrogel.4 (Table 1). The incorporation of bioactive peptides and growth factors via a heparin-binding delivery system improves the functionality of fibrin as a scaffold. New technologies such as inkjet printing and magnetically influenced self-assembly can alter the geometry of the fibrin structure and construct appropriate and predictable forms. Fibrin can be prepared from autologous plasma, and is available as glue or as engineered microbeads. No doubt from application point of view fibrin is a versatile scaffold for tissue engineering because it supports growth and development of various tissues in artificial cultures4 (Table 1).
Poly (L-lactic acid) scaffolds
Highly open porous biodegradable poly (L-lactic acid) scaffolds for tissue regeneration have been fabricated by using ammonium bicarbonate as an efficient gas foaming agent as well as a particulate porogen salt. A binary complex mixture of PLLA-solvent gel containing dispersed ammonium bicarbonate salt particles forms a paste that can be casted in a mold and subsequently immersed in a hot water solution to permit the evolution of ammonia and carbon dioxide within the solidifying polymer. It can be easily handled and molded into any shape, allowing for fabricating a wide range of temporal tissue scaffolds requiring a specific shape and geometry16 (Table 1). These interconnected macroporous scaffolds possess mean pore diameters of around 300-400 micron ideal for high-density cell seeding.
Porous silk scaffolds
Silk is used as a base scaffolding material to achieve sustainable cultivation of various biomaterials (Table 1). These 3-D silk protein scaffolds provide biocompatibility, porous features for transport, robust yet tunable mechanical properties. These can easily retain size and open porous structures for extended time frames due to slow proteolytic biodegradation, avoid specific cell signaling, and require no chemical cross-linking. Silk ionmer hydrogels and porous silk scaffolds have been made after making culture for up to 6 months. Silk degradation can be extended for months to years without premature collapse of structures (that would result in necrosis) to support cell interactions during slow remodeling toward native tissue. Silk can also be fabricated into different material formats, such as hydrogels, tubes, sponges, composites, fibers, microspheres, and thin films, providing versatile platforms and interfaces for a variety of different applications.2 Similarly, galactose containing physical cross-linked polyvinyl alcohol/gelatin hydrogel is formed that promotes cell-cell and cell-hydrogel interaction, aiding cellular aggregation leading to spheroids formation compared to void P/G hydrogel by 7 days.17 Thiol-norbornene (thiol-ene) photo-click hydrogels have emerged as a diverse material system for tissue engineering applications.8 These Thiol-norbornene hydrogels are prepared as tunable substrates for 2D cell culture, as microgels or bulk gels for affinity-based or protease-sensitive drug delivery. These are generated after cross-linking and follow degradation mechanisms and tunable material properties.18 (Table 1). These hydrogels are cross-linked through light mediated orthogonal reactions between multi-functional norbornene-modified macromers (e.g., poly (ethylene glycol), hyaluronic acid, gelatin) and sulfhydryl-containing linkers (e.g., dithiothreitol, PEG-dithiol, bis-cysteine peptides) using low concentration of photo-initiator.8 The gelation of thiol-norbornene hydrogels can be initiated by long-wave UV light or visible light without additional co-initiator or co-monomer.8 These hydrogels are used in drug delivery and have enormous tissue engineering applications8 (Table 1) (Figure 3).
Polycaprolactone (PCL) is also used as a framework material for scaffolding because it shows excellent mechanical properties.18 Collagen bioink is prepared which has different types of cells i.e. hepatocytes (HCs), human umbilical vein endothelial cells and human lung fibroblasts. These are infused into the canals of a PCL framework to induce the formation of capillary like networks and liver cell growth. Similarly, mLTs are developed which include liver microarchitecture and their in situ encapsulation in hydrogel composites.19 These mLTs encapsulated in collagen-alginate composites implanted into hepatic failure mice and sustained their survival during regeneration of the remaining liver.19 This act as a native liver microarchitecture and provides immune-protection without the need for complicated devices or processes. These nano devices are promising system for recovery of organ function.19
Polymer hydrogels
Hydrogel is composed of network of polymeric materials which form polymer chains hydrophilic in nature. These structures make hydrogels to hold large amounts of water in 3-D networks. Hydrogels are highly absorbent are capable of hold over 90% water in natural or synthetic polymeric networks. Numerous hydrogels have been developed based on natural and/or synthetic polymers and various kinds of crosslinking chemistry and have different biomedical applications.20 But based on the material used for polymerization and architecture and cross-link junctions hydrogels are of two main types chemical or physical. But on the basis of source biomaterial used these are of two types i.e. natural and artificial scaffolds. Natural hydrogels obtained from tissues were gradually replaced by synthetic types due to their higher water absorption capacity, long service life, and wide varieties of raw chemical resources. Physical and biochemical properties of hydrogels largely depend on their compositions, methods used for their polymerization, and their crosslinking density. No cytotoxic effects of GS-Hyd scaffold was found on the cells and Gelatin/Siloxane/Hydroxyapatite (GS-Hyd) scaffold was synthesized, degradation happened to the scaffold particles implanted into the muscle, followed by testicle and liver.21 (Figure 3).
Hydrogels are widely used in 3D culturing to study cell-matrix and cell-cell interactions, proliferation, migration,22 and controlled differentiation.23 Hydrogels are available in form of matrix, film, or microsphere, block diagrams that depends on optimized conditions maintained during the preparation process. Artificial hydrogels are designed to provide them a natural look, and resemble the characteristics of native extracellular matrix (ECM) (Figure 4). These provide three-dimensional (3D) supports for cellular growth and tissue formation.24 Hydrogels provide a versatile platform to include desired combinations of properties for designed applications(126).24–29 For different biomedical uses synthetic polymers such as gelatin methacryloyl (GelMA), methacrylated gelatin,30–33 methacrylamide modified gelatin34 or gelatin methacrylamide are used35,36 (Table 1). Most of them are least toxic to biological system.37,38 Hydrogel polymers also form colloidal gel in which water is the dispersion medium.
An innovative method for preparation of nanometal hydroxide superabsorbent hydrogel is polymer technology.39–41 Degradable natural polymer hydrogels are also fabricated for articular cartilage tissue engineering.42 Nanocomposites are also synthesized by incorporation of super paramagnetic Fe3O4 particles in negative temperature sensitive poly (N-isopropylacrylamide) hydrogels.43 Magnetic hydrogels have potential biomedical applications in drug delivery.44 and release, enzyme immobilization, cancer therapy and soft actuator.45 Semi-interpenetrating polymer networks are also made by using composed of poly NIPAAm and hydrophilic polymers.46 Cross-linking of poly (N-vinyl pyrrolidone) films is done by electron beam irradiation47 while synthesis of porous poly(N-isopropylacrylamide) gel beads by sedimentation polymerization.48 Chemically cross-linked networks form permanent junctions, while physical networks transient junctions. These arise either from polymer chain entanglements or physical interactions such as ionic interactions, hydrogen bonds, or hydrophobic interactions.49 chains, were used as a cell carrier for intravascular transplantation.50 It reduces shear stress and immediate immunological pressure after intravascular transplantation and provides biomatrix for environmental support. These cell-laden micro hydrogels provide long-term survival and engraftment efficiency to intravascular transplanted adult hepatocytes8 (Table 1). Similar, hydrogels (thermo-sensitive poloxamer gel and carbomer gel; hameln rds).51 with low [(125)I]-rhThrombin radioactivity are used for topical application. Chitosan, a copolymer derived from the alkaline deacetylation of chitin is also used for scaffolding (Table 1).
Polysaccharide hydrogels or porous and fibrous scaffolds
Polysaccharides are long carbohydrate molecules of monosaccharide units joined together by glycosidic bonds. These are complex biomolecules which are obtained from various sources i.e. animals, plants, microorganisms. These biological polymers show high biocompatibility, good availability and tailorable properties.52 These biopolymers are also used in form of starch, cellulose, chitosan, pectins, alginate, agar, dextran, pullulan, gellan, xanthan and glycosaminoglycans.53 These are used for engineering and regeneration of practically all tissues including blood vessels, myocardium, heart valves, bone, articular and tracheal cartilage, intervertebral discs, menisci, skin, liver, skeletal muscle, neural tissue and urinary bladder. These are also used for encapsulation and delivery of pancreatic islets and ovarian follicles. For therapeutic purposes polysaccharide based injectable hydrogels or porous and fibrous scaffolds are used with combination of other natural or synthetic polymers or inorganic nanoparticles. The immune response evoked by polysaccharides is usually mild, and can be reduced by purifying the material or by choosing appropriate crosslinking agents. Surface-attached fibrin structures containing extracellular matrix proteins improve adhesion and differentiation of endothelial cells on54 (Table 1) (Figure 4).
De-cellularization
Decellularization is used to isolate the extracellular matrix (ECM) of a tissue from its inhabiting cells. Decellularization of tissues or organs can provide an efficient strategy for preparing functional scaffolds for tissue engineering. Microstructures of native extracellular matrices and their biochemical compositions can be retained in the decellularized matrices (Figure 5), providing tissue-specific microenvironments for efficient tissue regeneration. This process is widely used in biomedical engineering to have ECM scaffold of the original tissue that can be used to generate engineered artificial organ and tissue regeneration. It reduces the chances of rejection of organ transplantation caused by antibodies.55 Tissue transplantation based on decellularization and formed organ is used to treat end organ failures. First of all Stephen F. Badylak established the process of decellularization and open the way to create natural biomaterial to act as a scaffold for cell growth, differentiation and tissue development. By recellularizing an ECM scaffold with a patient’s own cells, the adverse immune response is eliminated. A natural ECM scaffold provides the necessary physical and biochemical environment to facilitate the growth and specialization of potent progenitor and stem cells. Nowadays, commercially available ECM scaffolds are available for a wide variety of Tissue engineering. Moreover, de-cellularized matrix is required for both 2D coating and 3D hydrogel in liver tissue engineering.56 In case of severe morbidity of liver or hepatic failure only definitive treatment is orthotopic transplantation. However, due to donor organ shortage alternative technologies are emerged as whole organ decellularization methods. This could fulfill organ shortage and provide the ideal transplantable scaffold with all the necessary microstructure and extracellular cues for cell attachment, differentiation, vascularization, and function.3 But human hepatocyte transplantation is also used as alternative to liver transplantation.57 (Figure 5).
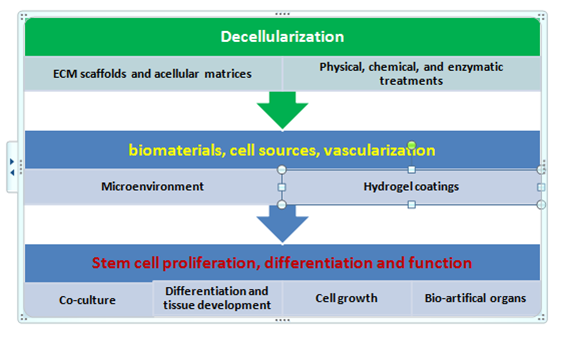
Figure 5 Showing process of de-cellularization and vascularization of tissues for generation of bio-artificial organs.
By using microstructures of native extracellular matrices and their biochemical compositions can be retained in the decellularized matrices which provide tissue-specific microenvironments for efficient tissue regeneration. For various biomedical use acellular matrices are isolated in vitro and in vivo in a number of different tissues and organs.58 More often, liver extracellular matrix (LEM) is used for two-dimensional (2D) coating and three-dimensional (3D) hydrogel platforms for culture and transplantation of primary hepatocytes. Similarly, LEM hydrogel coating enhanced mechanical properties and biological performance. Cell viability, differentiation, and hepatic functions. LEM hydrogel exhibited improved elastic properties, rapid gelation, and volume maintenance compared to Col I hydrogel. LEM coating significantly improved hepatocyte functions such as albumin secretion and urea synthesis and up regulated hepatic gene expression of human adipose-derived stem cells. The viability and hepatic functions of primary hepatocytes were also significantly improved in LEM hydrogel compared to Col I hydrogel both in vitro and in vivo. LEM is used to give functional biomaterial platforms for diverse applications in liver tissue engineering by promoting survival and maturation of hepatocytes and hepatic commitment of stem cells.56 (Figure 5). The development of artificial tissues require nutrients and oxygen that to be delivered via perfusion instead of diffusion alone over a short time period. One approach to perfusion is to vascularize engineered tissues, creating a de novo three-dimensional (3D) micro vascular network within the tissue construct. This significantly shortens the time of in vivo anastomosis, perfusion and graft integration with the host.59
For decellularization various combinations of physical, chemical, and enzymatic treatments are carefully given to isolate ECM scaffold. These treatments help to maintain the structural and chemical integrity of the original tissue.6 These ECM scaffolds are used to reproduce a functional organ by introducing progenitor cells or adult stem cells, (ASCs), and allowing them to differentiate within the scaffold to develop into the desired tissue. The produced organ or tissue can be transplanted into a patient. In contrast to cell surface antibodies, the biochemical components of the ECM are conserved between hosts, so the risk of a hostile immune response is minimized.60,61 Proper conservation of ECM fibers, growth factors, and other proteins is imperative to the progenitor cells differentiating into the proper adult cells. The success of decellularization varies depends on types of components present in biological tissue and density of associating cells and adhesion factors62 ECM scaffold maintains the protein and growth factors of the natural tissue. Decellularizing process is used to have biomaterial scaffolds for tissue regeneration of cardiac, dermal, pulmonary, renal, and other types of tissues. The most applicable success from decellularized tissues has come from symmetrical tissues that have less specialization, such as bone and dermal grafts. Decellularized tissue put inductive effect on microenvironment during differentiation of new cell types from stem cells in culture.63 Hence, to engineer bio-artificial whole organ perfusion-decellularized matrix is used as a natural 3D scaffold.64,65 This technology used to engineer lungs,66 tissue grafts for heart,67 liver68 and pancreas in vitr.69 Decellularization is also used in intestinal, renal and, cardiac, hepatic, skin tissue engineering (Figure 5).
All different methods used in decellularization do disruption of the architecture and potential loss of surface structure and composition. But it is very difficult to preserve complex composition and three-dimensional ultra structure of the ECM during decellularization. Physical methods and chemical and biologic agents are used in combination to lyse cells, followed by rinsing to remove cell remnants. Effective decellularization methodology is dictated by factors such as tissue density and organization, geometric and biologic properties desired for the end product, and the targeted clinical application.6 For cell lysis sodium dodecyl sulfate (SDS) an ionic detergent is commonly used. SDS shows high efficacy for lysing cells without significant damage to the ECM. Normally, detergents act effectively to lyse the cell membrane and expose the contents to further degradation. After SDS lyses the cell membrane, genetic contents are degraded by endo and exonuclease enzymes, while other components of the cell is solubilized and washed out of the matrix. Enzymes used in decellularization treatments are used to break the bonds and interactions between nucleic acids, interacting cells through neighboring proteins, and other cellular components. Few important enzymes like thermolysin, trypsin, galactosidase, nucleases are used for removal of cells.64 (Figure 5).
In addition, transplantable re-cellularised liver grafts are also developed by preparing a decellularised whole-liver scaffold. For decellularization and recellularization of a liver scaffold a chemical detergent was used good reproducibility.70 For preparing decellularised scaffold D-Hanks' solution containing sodium EDTA, gradient sodium dodecyl sulphate (SDS, 1/0.5/0.1%) and Triton X-100 were infused via the portal vein. HepG2 and C3A cells were seeded onto the scaffold, and a circulation perfusion culture was carried out.70 New scaffold are developed which promote expression of liver-specific functions in hepatocytes by immobilizing growth factors onto an organ-specific matrix for liver tissue regeneration.71 Solubilized extracellular matrix from decellularized liver (L-ECM) is obtained following Triton X-100 treatment and consisted of protein and polysaccharide. L-ECM was found to immobilize hepatocyte growth factor HGF), even in the presence of albumin, with an efficiency of 75%. This immobilized HGF promotes hepatocyte migration, and L-ECM-immobilized HGF maintained its native biological activity. L-ECM stimulated the expression of liver-specific functions, including albumin secretion, urea synthesis and ethoxyresorufin-O-deethylase activity, in primary rat hepatocytes cultured in growth factor-free medium.71 For optimization of the capture of in vivo hepatocytes from their microenvironment scalable bioartificial liver modular devices have been made. These devices are integrated nanostructured self-assembling peptides and a special combination of growth factors and cytokines. It lead to establish an in vivo type hepatocyte microenvironment pattern in vitro for predicting the in vivo drug hepatotoxicity.72 Contrary to this, undefined extracellular matrices like matrigel, rigid collagen, or serum supplementations, were found problematic and remain unacceptable for clinical applications.72 In addition, an ex vivo cellular model is also used in a bioreactor based liver module, a micropatterned module, a microfluidic 3D chip, coated plates, and other innovative approaches for the functional maintenance of primary hepatocytes72 (Figure 5).
Tissue vascularization and integration
Tissue vascularization and integration with host circulation is an important aspect and a key barrier to the translation of engineered tissues into clinically relevant therapies.73 Vasculogenesis is a process of differentiation undertaken by mesodermal cells to form new blood vessels. It completes in three ways i.e. differentiation of mesodermal cells into angioblasts or hemangioblasts; differentiation of angioblasts or hemangioblasts into ECs; and the organization of new ECs into a primary capillary plexus. In extrinsic vascularization methods, vascularization of engineered constructs is promoted ex vivo through material design, culture conditions, and cell source. Scaffolds sufficiently pre-vascularized ex vivo would be transplanted in vivo and encouraged to integrate with the host vasculature. This approach utilizes various biochemical signals embedded in scaffolds to mimic natural microenvironment and aims to maximize angiogenic potential of the seeded cell types. Sufficient neovascularization in scaffold materials can be achieved through coordinated application of angiogenic factors with proper cell types in biomaterials.74 The scaffolds are often modified with ECs co-cultured with secondary supporting cells, ECM proteins and peptides and angiogenic factors delivered along with vascular cell types. Prevascularization both in vivo and in vitro can be achieved by maintaining co-cultures of endothelial cells with the cell type of interest. These techniques can provide functional vasculature throughout the construct, though issues of cell culture time and functional anastomosis with host vessels remain a concern. Modular assembly is also done to make minimal functional units (e.g., an endothelial cell-coated hydrogel or a microtube-hydrogel composite system) and to build up a larger vascular network. Finally, in vivo systems such as polysurgery techniques or arteriovenous (AV) loops utilize the natural angiogenic potential of an organism to vascularize engineered tissues within the body (Figure 3).
The major hurdle in development of more complex tissues lies in the formation of vascular networks capable of delivering oxygen and nutrients throughout the engineered constructs. However, for this purpose multicellular tissue integration is used to engineer vascular architecture.73 Thus a micro tissue molding approach is used to make constructs containing highly aligned cords of endothelial cells triggered the formation of new capillaries along the length of the patterned cords. Silk-based degradable scaffolds biomaterials showed remarkable vascularization capacity and are used in soft tissue engineering and regenerative medicine.75 These biomaterials are mainly noncrystalline, offering improved cell proliferation than previously reported silk materials. These systems also have appropriate softer mechanical property that could provide physical cues to promote cell differentiation into endothelial cells, and enhance neo vascularization and tissue in growth in vivo without the addition of growth factors.75 Alginate scaffolds provide a favorable microenvironment for liver neo-tissue recreation and regeneration.57
Tissue engineering has some pervasive problems i.e. scaffold functionalization, modular assembly, cell-based techniques, bioreactor designs, micro electromechanical systems (MEMS)-related approaches, and delivery of nanoscale devices and molecules in vivo systems. Scaffolds can make functionalize through different angiogenic factor–loading techniques or through increased porosity or channeling of scaffolds to form perfusion elements. Moreover, use of vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and heparin-impregnated scaffolds or laser-cored porous scaffolds76–78 induce angiogenesis in vivo and reduce oxygen and nutrient gradient formation. Bioreactor systems such as perfusion systems, rotating systems, and spinner flasks specially are designed and employed to induce growth in tissues in vitro. These systems result in improved transport and can result in enhanced rate and quality of tissues generated in vitro. The use of MEMS and microfluidic technologies to recapitulate the branching network of the microvasculature is an alternative approach. But these systems are generally based on non degradable materials such as silicon or polydimethyl siloxane (PDMS). In addition, degradable systems are also fabricated with defined reproducible protocols.
Cell-printing methods
Cell-printing methods have been widely used in tissue regeneration because they enable fabricating biomimetic 3D structures laden with various cells. This is based on a multi-head tissue/organ building system is used for printing of a 3D cell-laden construct. This technology is also used to liver tissue engineering and improves poor mechanical properties of cell-laden hydrogels18 (Table 1). Polycaprolactone (PCL) is used as a framework material because of its excellent mechanical properties18 Collagen bioink containing three different types of cells-hepatocytes (HCs), human umbilical vein endothelial cells, and human lung fibroblasts--was infused into the canals of a PCL framework to induce the formation of capillary like networks and liver cell growth. A co-cultured 3D microenvironment of the three types of cells was successfully established and maintained.18 HCs showed vascular formation and functional abilities and are used in cell printing technology for the creation of heterotypic cellular interaction within a structure for liver tissue engineering.18
However, to achieve cell-matrix block, various natural hydrogels that are nontoxic, biocompatible, and printable have been combined to obtain "bioinks." Most bioinks, include alginates and show low cell-activating properties. Hence, for printing 3D porous cell blocks a new strategy is developed to have highly bioactive ink. It consists of collagen/extracellular matrix (ECM) and alginate. An in vitro assessment of the 3D porous structures laden with pre osteoblasts and human adipose stem cells (hASCs) demonstrates that the cells in the bioinks are viable.18 Besides cancer angiogenic factors are also used in tissue-engineering strategies via polymeric delivery systems in order to ultimately better mimic the stem cell niche through scaffolds. These polymeric vehicles are made of synthetic and/or natural biomaterials as scaffolds (Table 1). These are used for three-dimensional cell cultures and for locally delivering the inductive growth factors in various formats to provide a method of controlled, localized delivery for the desired time frame and for vascularized tissue-engineering therapies. In last two decades translational research has progressed to make non modular tissue constructs such as skin, bladders, vessels and upper airways.13 For their development, autologous cells are seeded on either artificial or natural supporting scaffolds.13 These constructs are implanted without the reconstruction of the vascular supply, and the nutrients and oxygen as they receive supply from adjacent tissues by diffusion. Models of functioning hearts and livers have been engineered using "natural tissue" scaffolds and efforts are underway to produce kidneys, pancreas and small intestine.13 But these modular organs also require vascular supply that is too complex and challenging.
Cross linking of biomaterials
3D hepatocellular spheroids aids in the maintenance of liver-specific functions in hepatocytes.79 Primary hepatocyte is probably the preferred cell for cell therapy in liver regeneration. However, its non-ideal proliferation capacity and rapid loss of phenotype during 2D culture compromises the quality and quantity of the transplanted hepatocytes, resulting in variable success rates of this treatment. For widening the clinical applications dual-functioning genipin cross-linked gelatin microspheres are which serve as cell carriers as well as porogens for delivering the model cells and also for creating cavitie.79 The cells were first seeded onto genipin cross linked gelatin microspheres for attachment, followed by encapsulation in alginate hydrogel.79 Collagenase, MMP-9, is used either in the culture media or mixed with alginate precursor solution to allow microsphere degradation for creating cavities within the gel bulk. Accordingly, the cells proliferate within the cavities, forming hepatocellular aggregates while the alginate hydrogel serves as a confinement, restricting the size and the shape of the aggregates to the size of the cavities.79
Statins also showed therapeutic effect of on bone formation and neo vascularization. But its low dose systemic administration is limited because it causes adverse side effects on liver metabolism and clearance in the digestive system. However, to avoid low-efficacy/frequent side effects of high-dose statin treatment, biodegradable gelatin hydrogel is used for drug delivery for fracture healing. Local administration of either simvastatin-conjugated gelatin hydrogel (ST-Gel group) or gelatin hydrogel alone (Gel group) in experimental animals induce functional bone healing significantly with increased angiogenesis- and osteogenesis-related growth factor expressions in periosteal granulation tissue.80 Collagen hydrogel is used as a matrix to generate engineered hepatic units to reconstitute three-dimensional, vascularized hepatic tissue in vivo. The engineered hepatic tissue has the ability to respond to the regenerative stimulus. Moreover, large hepatic tissue containing blood vessels could be engineered in vivo by merging small hepatic units. This approach for tissue engineering is simple and represents an efficient way to engineer hepatic tissue in vivo.81
Stem cell-based tissue engineering is highly applicable for the treatment of liver diseases and as drug metabolism and toxicity models in drug discovery and development.10 It is also used to replace defective body cells82 and improve local tissue defects.83 Cell therapies initially involved autologous bone marrow cell infusion, but methods for use of specific cells such as mesenchymal stem cells and macrophages have been developed.84 However, for derivatization of tissue specific stem cells, setting of micro-niche or environment is highly important. It essentially needs secretion of cell regenerative factors, growth factors, nutrients and matrix components. Use of scaffolds/hydrogels in cell culture medium regulates cell formation, differentiation, growth and function. Extracellular matrix plays a central role in regulating stem cell commitment. Cellular microniche composed by using all required growth factors decide cell division pattern, whether a stem cell divide symmetrically of asymmetrically. There are few somatic specific cell types that are involved in the organ's intrinsic regenerative ability. In addition, few exogenous cells - such as progenitor cells, embryonic stem cells, induced pluripotent stem cells, and bone marrow, adipose- and umbilical cord blood-derived stem cells and mesenchymal stem cells also contribute in repairing and regeneration in the absence of an innate intrinsic regenerative capability.
Mesenchymal stem cells (MSCs) are promising cell types that possess long life-span, facile isolation, rapid proliferation, prolonged transgene expression, hypo-immunogenicity, and tumor tropism.85 These cells show multiple differentiation abilities, and migrate directly into injured tissue and differentiate into hepatocyte-like cells.86 MSCs secrete various growth factors and cytokines before and after differentiation. These molecules increase hepatocyte regeneration, suppress liver fibrosis; regulate inflammation and immune responses.86 Cytokines involve in innate immunity while growth factors regulate liver regeneration. These factors are also secreted by undifferentiated and hepatocytic differentiated MSC in vitro and proved highly useful for acute and chronic liver diseases.87 MSCs exert their therapeutic effects by releasing stress-induced therapeutic molecules after their rapid migration to damaged tissues. MSC also secrete hepatotropic factors when implanted and potentially support liver regeneration.87 However, for improving therapeutic efficacy, genetically engineered MSCs have been developed for transgene expression by viral gene transduction and non-viral gene transfection.87 These engineered MSCs assist in making self-assembling supramolecular hydrogels, which assist in cell formation, their adhesion and are good for tissue regeneration.87 Mesenchymal stem cells are used for development of laboratory based 3D organs and tissues.14
However, in regeneration and tissue engineering both mesenchymal stem cell (MSC) therapy and a combination therapy of MSCs transfected with vascular endothelial growth factor (VEGF) are used for liver regeneration after major resection.88 Transplanted stem cells and MSCs transfected with VEGF significantly accelerate the healing process following major hepatic resection. However, an injection of MSCs and VEGF-transfected MSCs is given into the portal vein following liver resection. These cells are engrafted in the liver where they enhance proliferation of bile duct and liver hepatocyte. Injected MSCs secrete many growth factors including HGF, TGFβ, VEGF, PDGF, EGF, and FGF via paracrine effects which support liver function, regeneration, and liver volume/weight.88 Similarly, several growth factors and cytokines secreted in the conditioned medium from bone marrow-derived mesenchymal stem cells (MSC-CM) effect cell behavior. These factors are cytokines such as insulin-like growth factor-1, vascular endothelial growth factor, transforming growth factor-β1, and hepatocyte growth factor that occurs relatively in low amounts.89 These cell secreted factors cut down inflammation and show osteogenic potential by increasing cell mobilization, angiogenesis, and osteogenesis in vitro and in vivo.89 Similarly, MSC from adipose tissue and bone marrow expressed a similar pattern of secreted factors and surface markers. ASCs secrete multiple factors i.e. IFNγ and hepatocyte growth factor (HGF) which display unique action on epithelial cell hierarchy. IFNγ increased stem/progenitor-like cells while simultaneously reducing the size of mammospheres, whereas HGF increased the size of mammospheres with an accompanying increase in luminal progenitor cells. ASCs expressed higher levels of HGF, whereas adipocytes expressed higher levels of IFNγ.90 ASC co-culture or treatment with ASC conditioned media altered the number of CD49f (high)/EpCAM(low) basal/stem-like and CD49f(medium)/EpCAM(medium) luminal progenitor cells. It enhance hepatocytic differentiation and secretion of CD54 (intercellular adhesion molecule 1, ICAM-1) but secretion of CD166 (activated leukocyte cell adhesion molecule, ALCAM) decrease.90 Similarly, adipose-derived stem cells (ASC) are transplanted after reconstructive surgery after tumor ablation.91 Similarly, a single intravenous administration of stem cells derived from human exfoliated deciduous teeth (SHEDs) or of SHED-derived serum-free conditioned medium (SHED-CM) resulted in fibrotic scar resolution.91 This inhibits CCl4-induced hepatocyte apoptosis.91
Induced pluripotent stem cells (iPSCs) are also used as diagnostic and potentially therapeutic tools to study occurrence of model disease and assess the toxicity of pharmaceutical medications.92 These are also used for production of autologous stem cells from terminally differentiated somatic cells.79 Similarly, combinatorial extracellular matrix microenvironments promote survival and phenotype of human induced pluripotent stem cell-derived endothelial cells in state of hypoxi.93 Induced pluripotent stem cells (iPSCs) are used to treat acute hepatic failure (AHF) which is a severe liver injury leading to sustained damage and complications. However, hepatocyte-like cells (iPSC-Heps) are developed by reprogramming of human dental pulp-derived fibroblasts which convert into iPSCs. These iPSC-Heps resembled human embryonic stem cell-derived hepatocyte-like cells in gene signature and hepatic markers/functions. These specific cell types exhibit pluripotency and the capacity to differentiate into tridermal lineages.94 Further, HGF mediates the enhancement of iPSC-Hep antioxidant/antiapoptotic capacities and hepatoprotection. It is considered an excellent vehicle for iPSC-Hep engraftment in iPSC-based therapy against acute hepatic failure.94 Hepatocyte-like cells are derived via induced pluripotent stem cell (iPSC) technology by growing in either bioplotted PLLA collagen or matrigel sandwich control culture92 and mature on 3D extracellular matrix scaffolds. No doubt, these iPSC-derived hepatocytes cultured within both scaffolds remain viable, become polarize, and form bile canaliculi-like structures. Moreover, cells grown within ECM scaffolds had significantly higher P450 (CYP2C9, CYP3A4, CYP1A2) mRNA levels and metabolic enzyme activity.92
Stem-cell-derived hepatocyte transplantation is considered as a potential method for the therapy of acute and chronic liver failure. However, for formation of hepatocyte-like cells (iHeps) from mouse embryonic stem cells (mESCs) a new system was developed for sustained delivery of growth factors by using polyethyleneimine-modified silica nanoparticles. Nanoparticle coated growth factors promote embryonic stem cell differentiation, liver regeneration,92 and improves the expression of endoderm and hepatocyte-specific genes and proteins significantly. It also generates a higher population of functional hepatocytes in vitro. When these cells were transplanted into injured-liver of mice after four weeks, these mESC-derived cells have shown higher integration efficiency into the host liver and significantly restored the injured liver. Similarly, subretinal transplantation of hADSCs in RCS rats effectively delayed the retinal degeneration, enhanced the retinal cell survival and improved the visual function.95
For effective regeneration of tissues MSCs need long term expression of genes including Oct3/4, Nanog, and Sox2, 3-D culturing, and differentiation of cells and formation of cell layer. More-specifically endogenous cellular factors contribute a lot to tissue engineering and repair but cells also efficiently utilize exogenous sources to substitute for the organ's lost structure and/or function(s). The immobilization or delivery of growth factors has been explored to improve vascularization capacity of tissue engineered constructs; however, the use of growth factors has inherent problems such as the loss of signaling capability and the risk of complications such as immunological responses and cancer75 (Table 2). Several cytokines, chemokines, and growth factors maintain micro- and niche play crucial roles in HPC activation and differentiation.96 Fibroblast growth factors (Fgfs) regulate critical biological processes such as embryonic development, tissue homeostasis, wound healing, and tissue regeneration.97 Similarly, HB-EGF and HGF used to repair cholestatic liver injury induced by bile duct ligation (BDL). Combination of the growth factors exerts potent antioncotic (antinecrotic), antiapoptotic, anticholestatic, and regenerative effects on hepatocytes in vivo.98 By recruitment of intrinsic stem /progenitor cells in to the damaged sites can start regenerative activity and can make disable organs functional.99 Similarly, induced neural cells may restore the memory in memory related diseases. For this purpose cell cycle machinery should be uplifted and continued for synchronizing differentiation of implanted tissues and its proliferation in recipients. Moreover, RA and Wnt signaling as key regulators of HSC development can provide new molecular insights for developing much advanced clinical therapeutics of disables.100 But it is questionable that how long individually tailored stem cells can be used for therapeutic purposes will be new innovative areas of future research.101 There are new avenues to be open in the field of articular cartilage engineering and nano scaffolds and therapy in liver tissue engineering.102–104
Gene/Factors Family |
Action |
Regulatory Function |
Sox gene family |
(Sox1, Sox2, Sox3, and Sox15) Induction |
Crucial transcriptional regulators |
Klf Family |
Klf1, Klf2, Klf4, and Klf5 |
Transcription factors Regulatory protein |
Oct" family (Oct-3/4 Oct1 and Oct6) |
Induction |
Crucial transcriptional regulators |
Oct-4 (+) |
Pluripotency |
Differentiation |
Myc family (c-myc, L-myc, and N-myc) |
Proto-oncogenes |
Induction of iPS cells |
Glis 1 |
Transcription factor |
Induce pluripotency |
Nanog |
Promoting pluripotency |
Transcription regulatory protein |
LIN 28 |
mRNA binding protein |
Factor for iPSC generation |
hESC |
Pluripotency factor |
Lineage differentiation |
SSEA-3 |
SSEA-4, TRA-1-60, TRA-1-81, TRA-2-49/6E, and Nanog |
Markers specific to hESC |
SSEA-1, mESCs |
Inducer |
Mouse embryonic stem cell markers |
CHIR99021 |
Modulate TGF-β, Notch, MAPK signaling pathways |
promotes self-renewal in (m)ESCs |
Oct-3/4, Sox2, Nanog |
GDF3, REX1, FGF4, ESG1, DPPA2, DPPA4, and hTERT |
iPSCs expressed genes |
GCF Cytokines |
Different cytokines |
Inflammation, cystic growth, and bone resorption that characterize cystic lesions |
IL-3, GM.CSF, IL-6 |
Granulocyte (Phagocytic immature cells) |
Monocyte Macrophage precursors |
HSC, IL-3 and IL-6 |
Hematopoietic growth factors |
Antitumor agents for solid tumors |
Epo-SCF, GMCSF, IL-3 |
Erythropoietin receptor |
Erythrocyte progenitor Markers |
BMP TGFb |
Signaling protein |
Mesenchymal cell signaling pathways |
BMP, FGF, Hedgehog, Notch, PDGF, Wnt |
Bone regeneration factors |
Periosteal-mediated bone regeneration |
cTnT, cTnI and Cx43 |
Transcription factors |
Cardiac regenerative medicine |
bHLH |
Transcription factors MyoD, myogenin |
Myf5 and MRF4 Muscle regulator factors |
Drosophila BCL6 homolog |
Transcription factors |
Ken and Barbie promotes somatic stem cell self-renewal in the testis niche |
Let-7-Imp axis |
Regulates ageing of Drosophila |
Testis stem cell niche |
Calcimimectic |
R-568 regulating osteogenesis in oAFMCSs |
Promote bone regeneration |
cyclin D1-3 |
Control TGF-b Smad2/3 pathway |
Proliferation of developing tissues |
pSmad8 with pSmad1 and pSmad5 |
BMP signaling hfSCs regulation |
Hair morphogenesis |
Pax3 |
Transcription factor |
Alveolar rhabdomyosarcoma |
SF/HGF |
Paracrine cellular growth |
promotes proliferation, adhesion and survival of HPCs(CD34+) |
Telomerase reverse transcriptase |
Telomerase activity |
Form telomerase protein complex to sustain cell division |
GDNF TGF β Hedgehog |
Facilitates maturation |
Mesenchymal stem cell |
BFG |
Maintains calcium homeostasis |
Stimulating calcium efflux |
Transferring growth factor α |
TGF α |
Cytokine growth, development, inflammation |
VEGF |
Vascular endothelial growth factor |
Vasculogenesis and angiogenesis |
BDNP |
Brain-derived neurotrophic factor |
TrkB signaling Effectuate information processing, learning, and memory |
Table 2 Regulatory functions of different genes and growth factors in tissue repairing and regeneration.
For successful regeneration therapies these is a need of searching easily available natural biomaterials for development of new safer scaffolds, cell adhesion molecules, hydrogels and cellular growth factors and fine candidate molecules responsible for cell programming. But obtaining biomaterials and in vitrodevelopment of organs and tissues is not an easy task. It is challenging and costliest un-imaginable affair to take upon structure and function of an organ in culture. Though, few successes have been obtained related to repairing of tendons, cartilages, skeletal muscles, liver tissues and in bone regeneration. But new promising biomaterials and methods are highly demanded for therapeutic purposes to restore organ/tissue and cell level deformities. Meanwhile stem cells (MSCs) are implanted or injected into damaged tissue while stem cell progenitors are used to replace defective cells. However, for development of novel treatments, newer tissue repairing strategies and both biological and synthetic solutions are being made available for providing advanced clinical aids to the patients. In addition, inherently suitable scaffolds and matrices are to be generated for tissue engineering, stem cell propagation and differentiation. Now it become possible to use bio-engineered organs and tissues, though its replacement is very difficult to proceed and practiced for disables. There are so many issues related to regeneration of tissues remain unresolved and clinical challenges still exist despite the wide acceptance of the degenerative consequences of specific tissues. Therefore, there is an utmost requirement of new methods or strategies for having new potential regenerative medicine based on cell-free scaffolds, gene therapy, intra-articular delivery of progenitor cells, biological glues for joining broken bones, disarticulated tendons, muscle fibers, reparable tears, partial and total tissue engineered meniscus replacement. Further, regenerative medicines may also have important role in reprogramming of cells to check the ageing or maintain reversal of ageing process. But, it is a greatest challenge because during ageing, numerous intrinsic and extrinsic changes followed which influence stem-cell behavior and reduce tissue maintenance and regeneration. These mechanistic ageing-related changes in stem-cell behavior cannot be restored.
Tissue engineering is an inter-disciplinarily innovative research area which has wider applications repair of organs, regeneration and tissue transplantation. It has many clinical manifestations as by applying biological and tissue engineering methods self-sustained growth of degenerated or injured organ tissues could be achieved. Truly regenerative tissue rejuvenation has provided life to millions of accidental disabled persons across the world. Further, by supplying all transcription and growth factors in settling micro-niche expansion of stem cells desirable regenerated tissues can be obtained by using basic decellularized structures. By using latest technology and advanced scaffolds materials/hydrogels and ECMs regenerative medicine is fulfilling the demand and minimize the shortage of organs (donors) for transplantation. New advanced cell culture systems, media, regenerating growth factors, transcription factors and cell programming molecules have been explored which are used to develop new organs from decellularized matrix cell systems for replacement therapies. It is impossible to regenerate or rejuvenate the injured and degenerated organ whose repairing is impossible by using conventional drugs or by chemotherapeutics. By using tissue engineering methods large hepatic tissue containing blood vessels could be engineered in vivo by merging small hepatic units. Primary hepatocyte is probably the preferred cell for cell therapy in liver regeneration. In culture formation of 3D hepatocellular spheroids aids in the maintenance of liver-specific functions in hepatocytes For therapeutic purposes, polysaccharides have been applied in various forms, such as injectable hydrogels or porous and fibrous scaffolds, and often in combination with other natural or synthetic polymers or inorganic nanoparticles.
More specifically, for healing of defective or injured tissues synthesis of molecules, cell products, cells, and induction factors for regeneration of tissues present in human body is induced. Certainly, by exploring fine molecular mechanisms, signaling pathways of repair, process of replacing, restoration and regeneration of functionless vital organs can revitalize. With this safer scaffold materials, cell systems, methods and technologies are essentially needed to find solution of clinical failures of cell and tissue transplantation. Further, formation of various cell fusion types will reduce the rate of tissue degeneration and will assist from many glandular infections. Conceptually cells identical in physiological and structural regeneration of membrane surfaces and body metabolism can change the therapeutic approach in case of cancer and tumor. Hence, biologically active molecules which do immune-modulation and make transplantation corrections in tissue grafts are to be identified. Further, promising candidate molecules such as immunomodulators, adhesions, integrins and new biological scaffold materials are used for tissue repairing and induction of regeneration in injured tissues. No doubt, regenerative medicine is most emerging inter disciplinary field that has high biological, clinical and vast socio-economic importance is medicine of future. This innovative research area needs many conceptual improvements in glandular, cytoskeletal, neuronal and cardiovascular therapies.
None.
The author declares no conflict of interest.

©2017 Upadhyay. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.