Advances in
eISSN: 2572-8490


Research Article Volume 9 Issue 1
Correspondence: Deryabina OG, Institute of Genetic and Regenerative Medicine, M. D. Strazhesko National Scientific Center of Cardiology, Clinical and Regenerative Medicine, National Academy of Medical Sciences of Ukraine, Ukraine,
Received: October 20, 2023 | Published: November 7, 2023
Citation: Konovalov SV, Moroz VM, Deryabina OG, et al. Restoration of the nervous system in acute ischemia-reperfusion of the rat brain by intravenous administration of mesenchymal stromal cells of different origin. Adv Tissue Eng Regen Med. 2023;9(1):60-65. DOI: 10.15406/atroa.2023.09.00142
Transplantation of mesenchymal stromal cells (MSCs) is a promising direction in the therapy of ischemic stroke, which promotes the regeneration of neurons in experimental and clinical studies. We evaluated the neuroprotective and angioprotective properties of intravenously transplanted allogeneic and xenogeneic MSCs obtained from human and rat adipose tissue, human umbilical cord Wharton jelly and its cells lysate compared with citicoline in a rat brain ischemia-reperfusion model. We studied the somatosensory cortex and CA1 zone of the hippocampus in rats using immunohistochemical analysis. It was found that the CA1 zone of the hippocampus was the most sensitive to ischemic damage. Analysis of brain sections 7 and 14 days after ischemia-reperfusion showed that all treatment options increased the staining intensity of NeuN-positive neurons, but did not reach the control value in sham-operated rats. To quantify the obtained results, we used the integrated fluorescence density indicator, which demonstrated a decrease in the fluorescence intensity of NeuN-positive neurons in 4.2 (7 days) and 3.2 times (14 days). All treatment variants significantly increased the intensity of fluorescence, but human umbilical cord Wharton’s jelly MSCs gave the best result. There was no difference in the fluorescence intensity of RECA-1-positive blood vessels. Thus, immunohistochemical analysis using a broad panel of antibodies against neurons and endothelial cells demonstrated that ischemia-reperfusion resulted in the death of NeuN-positive neurons in the CA1 zone of the hippocampus. Transplantation of stem cells of different origin, lysate of umbilical cord Wharton’s jelly MSCs and administration of citicoline had a neuroprotective effect on the CA1 zone of the hippocampus. However, the best result has been shown by the treatment with Wharton’s jelly MSCs.
Keywords: ischemic stroke, neuroprotection, CA1 zone of the hippocampus, mesenchymal stromal cells, citicoline
MB, Mitochondrial diseases; mtDNA, mitochondrial DNA; MRI, magnetic resonance imaging; GTCS, generalized tonic-clonic seizure; HSV, herpes simplex virus; ENMG, electroneuromyography
Ischemic stroke is the leading cause of death and long-term disability among patients with acute cerebrovascular accident (ACVA) worldwide.1–4 After ACVA, patients throughout their lives bear the burden of various physical and mental disorders, social and economic difficulties, which, in turn, causes huge problems for the state and society.5
A deep understanding of the structural and morpho-functional changes in the affected area of the brain helps in finding targets for possible correction of the consequences of ACVA. Disruption of blood supply in the neurons of the ischemic area provokes irreversible changes in electrolyte homeostasis with the formation of necrosis centers and the formation of the infarct core zone.6 In the first hours, a penumbra zone (half-shadow) is formed around the infarct core, where there is still minimal blood flow due to collateral blood flow. Cells of the penumbra can subsequently undergo irreversible damage and, thus, increase the infarct zone or restore normal vital activity if perfusion is quickly improved.7 Reducing the intensity of the ischemic cascade through the protection of penumbra neurons is a promising direction of neuroprotective therapy.6
Cell therapy using mesenchymal stromal cells (MSCs) demonstrated encouraging results regarding endogenous mechanisms of neuroregeneration in response to ischemic damage of brain structures.9–14 The main source of MSCs is bone marrow,15 however, due to age-related degeneration and the risk of infection, allogeneic umbilical cord Wharton's jelly MSCs are more accessible for obtaining and safer for humans. Such MSCs can be received in large amount, they are multipotent, proliferative, hypoimmunogenic, do not induce oncogenesis and are able to exist for a long time in the host's brain. Their use caused regeneration of neurons in models of ischemia-reperfusion, but not migration or implantation of cells in the lesion.16 Intravenous transplantation of human MSCs 24 h after stroke reduced neuronal damage and functional deficits due to attenuation of post-ischemic inflammation.17 However, there are still no data of the comparative effectiveness of MSCs of different origin on the ability of neurons and blood vessels to regenerate. Below, we provide a comparative assessment of the neuro- and angioprotective properties of MSCs of different origin, MSC lysate and citicoline in rats with acute cerebral ischemia-reperfusion. The obtained data are a continuation of previously published results.18
Animals
All the experiments on animals were carried out in accordance with the methodological recommendations of the Ministry of Health of Ukraine and the requirements of bioethics regarding the National "General Ethical Principles of Experiments on Animals", approved by the First National Congress on Bioethics (Kyiv, Ukraine, 2001) and the Law of Ukraine "On the Protection of Animals from Cruelty" dated 26 February 2006. The selected model of ischemia-reperfusion (IR) described by A.A. Khodakivskyi,19 consisted in imposing bilateral ligatures on the internal carotid arteries under propofol anesthesia (Propofol-novo, Novofarm-Biosintez LLC, Ukraine, 60mg/kg) lasting 20 minutes. The experiment was performed on 190male Wistar rats aged 4 months and weighing 160-190g. Animals, vivarium of Vinnytsia National Medical University named after E. Pirogov, were kept in standard conditions with free access to water and food. MSCs transplantation was performed once at a dose of 1×106 donor cells per animal immediately after IR. The selected technique requires a smaller number of cells, also significantly reduces the lesion and contributes to the recovery volume.20–22
|
Animal group |
Animal number |
Treatment |
|
1st group |
10 |
pseudo-operated animals + 0.9% NaCl solution at a dose of 2 ml/kg |
|
2nd group |
40 |
IR+0.9% NaCl solution at a dose of 2 ml/kg |
|
3rd group |
20 |
IR+MSC from human Wharton’s jelly at a dose of 106 cells/animal |
|
4th group |
20 |
IR+rat fetal fibroblasts at a dose of 106 cells/animal |
|
5th group |
25 |
IR+MSC from human adipose tissue at a dose of 106 cells/animal |
|
6th group |
25 |
IR+stem cells from rat adipose tissue at a dose of 106 cells/animal |
|
7th group |
25 |
IR+MSCs lysate from Wharton's jelly in a dose of 0.2 ml/animal |
|
8th group |
25 |
IR+citicoline 250mg/kg |
Table 1 Distribution of animals in the experiment
The 1st group of pseudo-operated rats, which were sequentially subjected to the following interventions (anesthesia, skin incision, vessel preparation) with the exception of ligation of the internal carotid artery (ICA), which reproduced the effect of the traumatic conditions of the experiment. The 2nd group is a group with control pathology. Rats of this group were subjected to 20-minute ischemia of the brain by applying ligatures to the ICA. After 20 minutes, the ligatures from the ICA were removed (reperfusion) and a 0.9% NaCl solution at the rate of 2 ml/kg was injected once into the femoral vein. A similar dose was administered to rats of the 1st group. The 3rd group of animals immediately after IR was transplanted with 106MSCs/animal from human Wharton jelly. The 4th group of animals underwent a single transplantation of rat fetal fibroblasts at a dose of 106cells/animal immediately after IR. The 5th group of animals with IR received 106MSCs/animal from human adipose tissue. The 6th group of animals was injected immediately after IR with 106MSCs/animal obtained from rat adipose tissue. The 7th group of animals, immediately after IR, was injected with 0.2 ml/animal of MSCs lysate (MSCs isolated from human Wharton jelly). The 8th group of rats received a single dose of the reference drug citicoline (Neuroxon, Arterium Corporation, Ukraine) immediately after IR at a dose of 250mg/kg. The choice of the drug is due to its proven ability to enhance neuroregenerative processes in an experiment on rats and to improve cognitive and memory functions in patients with cerebral ischemia.23–26
To analyze the effects of MSCs of different origin, MSC lysate of human Wharton jelly cells and citicoline on the somatosensory area of the cortex and the CA1 zone of the hippocampus, the brains of rats were quickly removed after decapitation using pentobarbital anesthesia (Penbital, Bioveta a.s., Czech Republic, 100mg/ kg) after 7 days (subacute period of ischemia) and 14 days (recovery period) after IR.15, 27, 28
Immunohistochemical analysis of frontal brain slices
Before starting the immunohistochemical analysis, paraffin sections of the brains of experimental animals were deparaffinized in xylene, followed by washing of slides with sections in 0.1 M phosphate buffer (PB). From brains fixed in formaldehyde, which were not embedded in paraffin, 40-μm-thick frontal brain sections were made using a VT1000A vibratome (Leica, Germany). Paraffin or vibratome sections after washing in 0.1 M PBS were blocked in a solution of 0.1 M PBS (pH 7.4) with the addition of 0.5% bovine serum albumin (BSA) and 0.3% Triton X-100. Incubation with primary antibodies (Table 2) was performed for 48 hours at 4°C. Primary antibodies were visualized using secondary antibodies conjugated with fluorescent label Alexa Fluor® 488, 594 or 647 (1:1000, Invitrogen, USA). Stained sections were covered with Immu-MOUNT medium (Thermo Scientific, USA).
|
No. |
Name |
Manufacturer, catalog number |
|
1 |
Mouse anti-NeuN (NEUronal Nuclei), clone A60 |
SigmaAldrich, St. Louis, MO, USA, Cat. № МАВ377 |
|
2 |
Rabbit anti-GFAP (Glial Fibrillary Acidic Protein) |
DakoCytomation, Glostrup, Denmark, Cat. № Z0334 |
|
3 |
Mouse anti-RECA-1 (rat endothelial cell antigen), clone HIS52 |
AbD Serotec, Oxford, UK, Cat. № MCA970GA |
|
4 |
Donkey anti-mouse Ig (H+L) Alexa Fluor® 594 |
Invitrogen, Carlsbad, USA, Cat. № A21203 |
|
5 |
Donkey anti-mouse Ig (H+L) Alexa Fluor® 488 |
Invitrogen, Carlsbad, USA, Cat. № A21202 |
|
6 |
Donkey anti-rabbit Ig (H+L) Alexa Fluor® 594 |
Invitrogen, Carlsbad, USA, Cat. № A21207 |
|
7 |
Donkey anti-rabbit Ig (H+L) Alexa Fluor® 647 |
Invitrogen, Carlsbad, USA, Cat. № A31573 |
Table 2 Antibodies used for immunohistochemical analysis
Immunohistochemically stained brain sections were examined using an FV1000-BX61WI confocal scanning microscope (Olympus, Japan) using green helium-neon (HeNeG laser 543 nm), red helium-neon (HeNeR laser 633 nm) and multiline argon (Multi Ar laser 458, 488, 515 nm) lasers.
For each marker examined, the same magnification, exposure time, and other laser settings were used. The resulting images were used to quantify the fluorescence intensity of each marker. A total of 5 sections per animal were examined. The intensity and area of the fluorescent marker were measured by automatically calculating the average gray value within the measurement threshold. The results are presented as the integrated fluorescence density in conventional units (с.u), which is equal to the fluorescence intensity multiplied by the fluorescence area (without taking into account the integrated background fluorescence density).
Statistical analysis
To compare the results of experimental groups (1-8) study, the ANOVA method of variance analysis was applied using the following software: Statistica (version 6, Statsoft, USA), ImageJ (Wayne, Rasband, USA). The difference was considered significant at p≤0.05. Integrated fluorescence density in a frontal brain slice is shown as the mean value for 5 slices ± standard error of the mean (M±SEM).
Immunohistochemical analysis using neuron markers (anti-NeuN antibody) showed no differences between intact and sham-operated animals, so the group of sham-operated animals was used as a control. On the frontal slices of the brain of sham-operated animals, in the somatosensory cortex, NeuN-positive neurons were characterized by intense fluorescence and were located in layers that had different densities (Figure 1A).
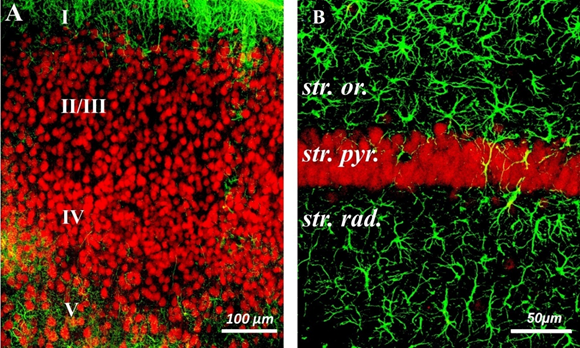
Figure 1 Confocal micrographs of frontal sections of the rat brain.
A-Somatosensory cortex of the brain. Cell layers are indicated by numbers.
B–CA1 zone of the hippocampus. Marked layers: st. or – stratum oriens, st. pyr. – stratum pyramidale, st. rad. - stratum radiatum. Double immunohistochemical staining. NeuN-positive neurons are colored red, GFAP-positive astrocytes are colored green. Scale bars: A = 100µm, B = 50µm.
Several scattered NeuN-positive neurons were detected in layer I. Small and medium pyramidal NeuN-positive neurons were observed in layers II/III. Dense cell layer IV contained different types of pyramidal and stellate NeuN-positive neurons. In layer V, large pyramidal NeuN-positive neurons and a network of well-branched GFAP-positive processes were visualized (Figure 1A).
NeuN-positive pyramidal neurons of the hippocampus were characterized by intense fluorescence and were located in the form of a dense layer of stratum pyramidale, which was formed by 3-5 layers of neurons (Fig. 1B).
After the immunohistochemical study of the frontal sections of the pseudo-operated animals, the next stage of the study was the analysis of the cytoarchitectonics of the rat brain after IR. Immunohistochemical analysis showed that the CA1 zone of the hippocampus was the most sensitive to ischemic damage. Changes in the somatosensory cortex and other areas of the brain were not statistically significant. Therefore, in further immunohistochemical studies, we focused our attention on the CA1 zone of the hippocampus.
On 7th and 14th days after IR, the fluorescence intensity of NeuN-positive neurons in the CA1 zone of the hippocampus significantly decreased (Figure 2A). The fluorescence intensity of the vast majority of NeuN-positive neurons in the CA1 pyramidal layer of the hippocampus (Figure 2B). was significantly lower than in the control group of sham-operated animals (Figure 1A). Immunohistochemical analysis showed that the number of intact NeuN-positive pyramidal cells that showed maximal fluorescent staining was negligible.
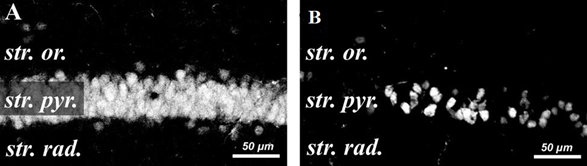
Figure 2 Confocal micrographs of NeuN-positive pyramidal neurons in the CA1 zone of the hippocampus.
A–pseudo-operated animal
B–animal after ischemia-reperfusion. Scale bar =50µm.
The next stage of immunohistochemical analysis was studying the influence of transplantation of different origin stem cells, MSCs lysate and citicoline on the state of nervous tissue after IR of the brain.
Immunohistochemical analysis of brain sections for the presence of the neuron marker NeuN showed that on 7 and 14 days after IR, all treatment options (groups 3-8) contributed to an increase in the staining intensity of NeuN-positive neurons compared to the 2nd group of animals (ischemia), but did not reach the control value of fluorescence in sham-operated rats (group 1) (Figure 3).
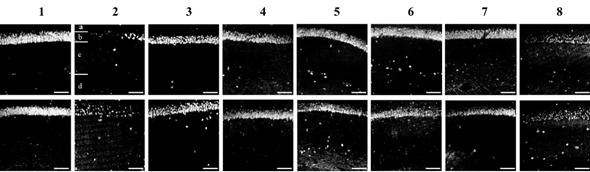
Figure 3 Confocal photomicrographs of frontal CA1 sections of the hippocampus zone, stained with the neuron marker NeuN.
The layers in the CA1 zone of the hippocampus are marked with letters: a – stratum oriens, b – stratum pyramidale, c – stratum radiatum, d – stratum lacunosum-moleculare.
The upper panel of photomicrographs is 7 days after ischemia-reperfusion, the lower panel is 14 days. Experimental groups (1-8) are indicated by numbers. Scale bar = 100 μm.
The integrated fluorescence density indicator was used to quantify the obtained results. The integral fluorescence density of NeuN-positive neurons on the frontal slices of the rat brain of different experimental groups is presented in Figure 4.
Statistical analysis of the integral fluorescence density of NeuN-positive neurons showed that IR reduced the fluorescence intensity in 4.2 (7 days) and 3.2 times (14 days) compared to group 1 (pseudo-operated animals) and was 6.86x106 ± 0.92 and 8.67x106 ± 0.89 c.u.
All treatment options (groups 3-8) demonstrated a statistically significant increase in fluorescence intensity compared to the 2nd group of animals (group IR). The use of human umbilical cord Wharton's jelly MSCs (group 3) showed the best result and the integrated fluorescence density of NeuN-positive neurons in this group was 21.07x106 ± 1.37 c.u. (7 days) and 23.48х106 ± 1.64 c.u. (14 days), but did not reach the values of the control group of pseudo-operated rats: - 28.79x106 ± 2.35 c.u. (7 days) and 27.84х106 ± 1.72 c.u. (14 days). Also, the integral fluorescence density of NeuN-positive neurons in group 3 was statistically significantly higher compared to citicoline (reference drug) both on the 7th and 14th day of the study.
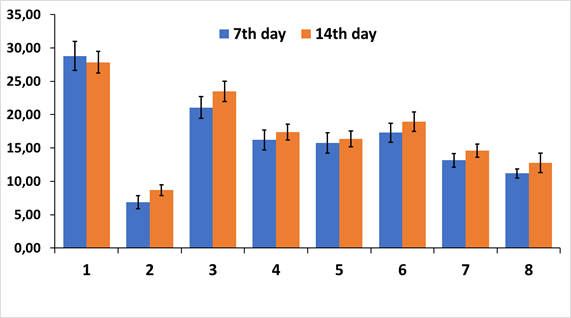
Figure 4 Integral density of fluorescence of NeuN-positive neurons on frontal sections of the CA1 zone of the hippocampus.
Experimental groups (1-8) are plotted on the abscissa. ordinate axis - conventional units (c.u.)
To investigate possible angioprotective properties of transplanted stem cells, an immunohistochemical analysis of CA1 endothelial cells of the hippocampus zone was performed using the RECA-1 (rat endothelial cell antigen) marker (Figure 5).
Immunohistochemical analysis of CA1 endothelial cells of the hippocampal zone showed that antibodies against RECA-1 stained the blood vessels of the hippocampus well, the borders of individual endothelial cells in RECA-1-positive blood vessels were clearly visualized (Fig. 5). RECA-1 reactivity with other cells, such as fibroblasts, leukocytes, and non-endothelial stromal cells, was not detected. Since previous immunohistochemical analyzes showed the best treatment result in group 3 (MSCs from human Wharton's jelly cells), we decided to compare only this group of experimental animals with sham-operated animals, group 2 (IR) and the reference drug citicoline.
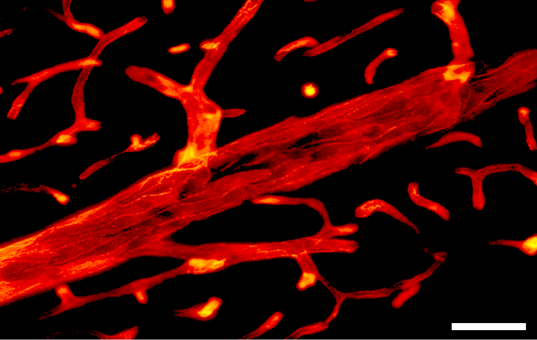
Figure 5 Photomicrograph of RECA-1-positive vessels in the CA1 zone of the hippocampus. Scale bar=50µm.
Immunohistochemical analysis of RECA-1-positive blood vessels on the 14th day after IR and MSCs from human Wharton's jelly transplantation demonstrated intense RECA-1 immunofluorescence in all layers of the CA1 zone of the hippocampus, but the most developed blood network was in the stratum lacunosum-moleculare layer (Figure 6).
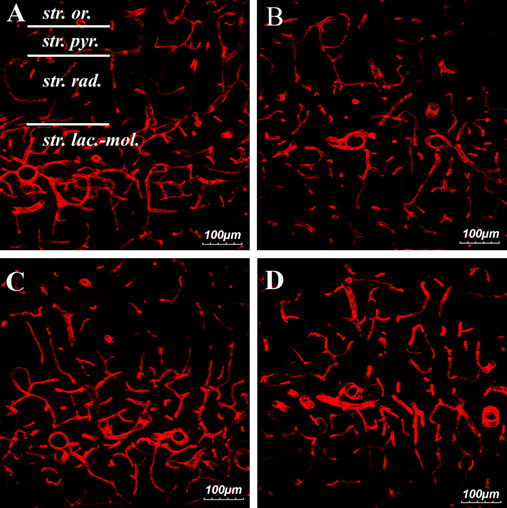
Figure 6 Confocal images of RECA-1-positive vessels in the CA1 zone of the hippocampus on the 14th day after ischemia-reperfusion.
A–pseudo-operated animal
B–ischemic animal
C–ischemic animal with transplanted MSCs from human Wharton’s jelly
D–ischemic animal with citicoline injection.
Designation of layers in the CA1 zone of the hippocampus: str. or – stratum oriens, str. pyr. –stratum pyramidale, st. rad. –stratum radiatum, st. lac.-mol. – stratum lacunosum-moleculare. Scale= 100µm.
Quantitative analysis of RECA-1 immunofluorescence showed that the difference in fluorescence intensity of RECA-1-positive blood vessels between the studied groups was not statistically significant (Figure 7).
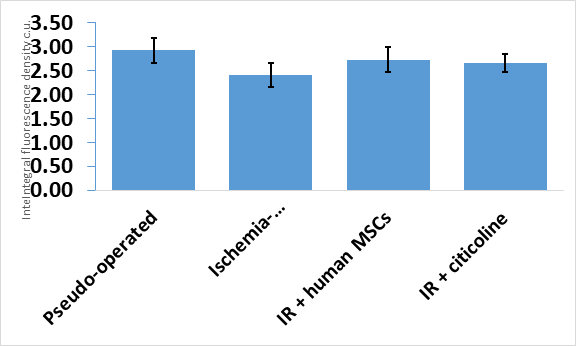
Figure 7 Integral fluorescence density of RECA-1-positive blood vessels in the CA1 zone of the hippocampus. Experimental groups are plotted on the abscissa axis.
Our study showed that allogeneic and xenogeneic transplantation of MSCs of various origin and citicoline in the IR model in rats reduce the volume of ischemic brain damage. The obtained data correspond to data,15, 29-31 which indicate that transplantation effectively protects ischemic penumbra neurons and has certain angioprotective properties. This is due to the ability of MSCs to secrete a wide range of trophic and immunomodulatory cytokines - the MSCs secretome,17, 30, 31 which has a protective effect on the ischemic brain in rats. This leads to the induction of endogenous neuroprotection, neurogenesis, angiogenesis, therefore MSC transplantation was recognized as safe procedure in preclinical studies and the recommended recovery strategy for treatment.32 The demonstrated neuroprotective properties of transplantation of stem cells of Wharton's jelly cells may indicate the prospect of their use in cell therapy of acute cerebral ischemia.
Thus, immunohistochemical analysis using a broad panel of antibodies against neurons and endothelial cells demonstrated that IR resulted in the death of NeuN-positive neurons in the CA1 area of the hippocampus. The use of all treatment options (transplantation of stem cells of various origin, lysate of umbilical cord Wharton's jelly MSCs, and the reference drug citicoline) had a neuroprotective effect on the CA1 zone of the hippocampus. The best result in the CA1 zone of the hippocampus was demonstrated by treatment with MSCs of umbilical cord Wharton's jelly compared to the reference drug citicoline.
None.
The authors declare that there are no conflicts of interest.
None.

©2023 Konovalov, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.