Advances in
eISSN: 2572-8490


Review Article Volume 9 Issue 1
Correspondence: Dr. Bill Tawil, Department of Bioengineering, UCLA School of Engineering, Los Angeles, USA
Received: September 11, 2023 | Published: October 9, 2023
Citation: Aryan P, Bill T. Nonalcoholic fatty liver disease: a review of physiology, treatments, and market outlook. Adv Tissue Eng Regen Med Open Access. 2023;9(1):42-48. DOI: 10.15406/atroa.2023.09.00140
The liver is a multifunctional organ with a wide variety of roles in the body; it is highly vascularized, and 25% of cardiac output is directed toward the liver. Non-alcoholic fatty liver disease (NAFLD is diagnosed when liver cells are injured as a result of fat buildup. People with NAFLD are at greater risk of having the disease progress to hepatis, cirrhosis, and liver cancer. The purpose of this review is to discuss the physiology, available treatments, and market for non-alcoholic fatty liver disease (NAFLD). NAFLD has increased in frequency around the world in the last 20 years, and this trend is predicted to continue into the future. The best known treatment for NAFLD is liver transplantation, but only a fifth of the patients who need treatment are provided with a donor liver. One alternative to a liver transplantation is the use of an artificial liver support system, which uses a variety of dialytic techniques to treat the blood plasma outside the body. Researchers are currently in the process of building bioartificial livers and chemically derived drugs to help reduce the effects of NAFLD, which can range from liver cancer to cirrhosis. While the list of treatments for NAFLD is limited, there is great potential for the industry to grow exponentially once additional treatments are approved by the Food and Drug Administration (FDA).
Keywords: nonalcoholic fatty liver disease, bioartificial liver, cirrhosis, nonalcoholic steatohepatitis, liver, hepatocytes, liver transplantation
NAFLD; nonalcoholic fatty liver disease; NASH, nonalcoholic stetaohepatitis; FDA, food and drug administration; NAFL, non-alcoholic fatty liver; HDL, high-density lipoprotein; ALP, alkaline phosphatase; DNL, de-novo lipogenesis; VLDLs, very low-density lipoproteins; TLRs, toll-like receptors; MARS, molecular adsorbent recirculating system; SPAD, single-pass albumin dialysis; MELS, modular extracorporeal liver system; FXR, farnesoid X receptor
The functional unit of the liver is the lobule, which is made of hepatocytes arranged in a hexagonal fashion around a central vein. Hepatocytes make up 80% of the cells in the liver, and they are constantly perfused with blood via the hepatic artery and portal vein. Hepatocytes constantly secrete proteins like albumin, plasminogen, fibrinogen, and clotting factors into the blood. Hepatocytes are all important for the production of bile. Synthesis of bile begins in the hepatocytes. The hepatocytes’ most important function, however, is their ability to remove toxins from the blood.
Non-alcoholic fatty liver disease (NAFLD) is a prevalent disease around the world, and the amount of people who suffer from the disease has increased steadily in the last 20 years. While most people who have NAFLD are asymptomatic, the risk of liver cirrhosis and non-alcoholic steatohepatitis (NASH) is significantly higher in people with the disease than in people who are otherwise healthy. This fact, combined with the fact that NAFLD has increased in frequency since the late 1990s, means that NAFLD-induced cirrhosis will become the leading cause for liver transplants by 2045. Currently, the most viable treatment for NAFLD is partial or complete liver transplantation, but healthy donors are limited and cannot adequately supply the total number of people who need a liver transplant.1 For those who cannot obtain a liver transplant, the next best option is for the person to depend on artificial liver support devices, which remove plasma from the body and filter it through a series of semipermeable membranes.2 In addition to artificial liver support devices, researchers and biomedical companies have developed bioartificial livers and a variety of drugs to treat the disease, but they still await FDA approval.8,21 This review paper will discuss the physiology of a healthy liver and a liver with NAFLD, as well as a discussion of the current and upcoming technologies that exist to treat the disease. Finally, this paper will discuss the future outlook of NAFLD treatment and how the industry will evolve as time passes.
The liver is a multifunctional organ with a wide variety of roles in the body, ranging from supporting the body’s metabolism and immune system to performing digestive functions and detoxification of the blood. While the liver only makes up 2% of the adult body weight, it is highly vascularized, and 25% of cardiac output is directed toward the liver. In contrast to other organs, which often receive their blood supply from a single major artery, the liver takes in blood from both the portal vein and the hepatic artery. 75% of the liver’s blood supply comes from the portal vein, while the other 25% comes from the hepatic artery.42 The liver can be divided into the left and right lobes, which can be described either by the morphologic anatomy of each lobe in the organ or by the functionality of the lobe in the liver.43 The left lobe of the liver is in charge of processing blood from the stomach and lower esophagus, and the hindgut, while the right lobe is in charge of processing blood from the midgut. A diagram of the liver and its lobes is shown in Figure 1. Figure 1 also shows eight segments of the liver, which are also characterized by differences in function.44
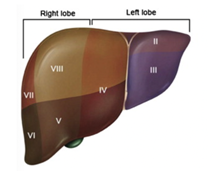
Figure 1 Depiction of left and right lobes of the liver, as well as the eight segments of the liver.44
The left lobe processes blood from the stomach, lower esophagus, and hindgut, while the right lobe processes blood from the midgut. The eight segments are also labeled on the figure, and each segment has its own blood supply and bile drainage system.51 The segments of the liver can be divided based on their functions, similar to how the lobes are designated.
The functional unit of the liver is the lobule, which is made of hepatocytes arranged in a hexagonal fashion around a central vein. Within each lobule, the hepatocytes are lined around the central vein. Between the vein and the cells are sinusoids, which contain Kupffer cells that serve as macrophages for the liver. Sinusoids also contain stellate cells, which are the fat cells of the liver. At the vertices of the lobule, there is a bile duct branch, a portal vein branch, and a hepatic artery branch, which together form a portal triad. Figure 2 shows the structure of a lobule as well as a depiction of how hepatocytes in different parts of a lobule have different functions in the body. Zone 1 hepatocytes, which are found right next to the portal triads, perform oxidative metabolism. Zone 2 hepatocytes, which are closer to the central vein, perform metabolic processes and interact with biological drugs 45. Zone 3 hepatocytes, which closely surround the central veins, are primarily involved with the transformation of medical drugs in the body.45
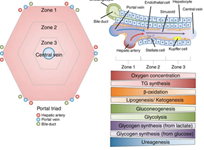
Figure 2 Depiction of a lobule and the portal triad, as well as the division of hepatocytes in Zones 1-3 and their functions.46
Zone 1 is closest to the portal triads, and hepatocytes there are in charge of performing oxidative metabolism. Zone 2 hepatocytes interact with biological drugs, and Zone 3 hepatocytes are closest to the central veins and interact with drugs in the body. The figure shows the division of functions within each zone, as well as the structure of hepatocytes around the hepatic artery and bile duct.
Hepatocytes make up 80% of the cells in the liver, and they are constantly perfused with blood via the hepatic artery and portal vein. As they are epithelial cells, hepatocytes contain apical and basolateral plasma membranes, which are crucial to their normal function. Hepatocytes constantly secrete proteins like albumin, plasminogen, fibrinogen, and clotting factors into the blood, and this is the reason why the liver releases more than 10 grams of albumin per day into the blood. In addition to protein secretion, hepatocytes are important for the production of bile, which is used to digest lipids. Synthesis of bile begins in the hepatocytes, and osmotic gradients allow the bile to be transported to the hepatic bile duct, where the bile is alkalized and diluted until it enters the gallbladder for storage. The hepatocytes’ most important function, however, is their ability to remove toxins from the blood. There are multiple mechanisms through which toxins can be processed, ranging from receptor-mediated endocytosis to fluid phase endocytosis.47
While there are a large variety of diseases that are associated with the liver, this paper will focus on non-alcoholic fatty liver disease, which encompasses any disease where adipose tissue accumulates in the liver. NAFLD can be characterized by non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH), and NASH is diagnosed when liver cells are injured as a result of fat buildup. People with NAFLD are at greater risk of having the disease progress to hepatis, cirrhosis, and liver cancer.48
There are a number of risk factors that increase a person’s risk of developing NAFLD. Metabolic syndrome, characterized by high triglycerides, low high-density lipoprotein (HDL) cholesterol, hyperglycemia, a large waist circumference, and hypertension, all significantly increase a person’s risk of getting the disease. People with type 2 diabetes mellitus also have, on average, 80% more liver fat than people who do not have the disease. Diet also plays a significant role in a person’s risk for developing NAFLD, as people who eat traditionally Western diets that are high in fats and sugars have a higher risk of obtaining metabolic syndrome. Polycystic ovarian syndrome, which is characterized by obesity and a resistance to insulin in women, also increases the risk of getting NAFLD.48
While the development of NAFLD is complicated and more research is needed to completely understand the pathophysiology of NAFLD, researchers have found that there are two types of NAFLD. The first type of NAFLD actually has a limited correlation with metabolic syndrome, and it is likely that insulin resistance is the primary contributor to a person’s risk of developing this type of NAFLD. The second type is associated with hepatitis C and HIV, as well as medications like glucocorticosteroids and vinyl chloride 49. Most patients with NAFLD do not suffer from any symptoms, but a few symptoms that people have can range from general fatigue to lipomatosis, which is the presence of fatty deposits throughout the body. When performing blood tests, an increase in aminotransferases (AST and ALT) is associated with an increased risk of a given patient having NAFLD.49 Elevated levels of alkaline phosphatase (ALP) also can indicate the disease. Figure 3 lists some indicators for the development of NAFLD in patients.
Figure 3 List of risk factors for NAFLD.49
A sedentary lifestyle and a high body weight are the most common indicators for NAFLD. In many cases of NAFLD, patients consume a diet consisting of large amounts of simple carbohydrates, a large caloric intake, and a large sodium intake. Stress and smoking also increase the risk of getting NAFLD. An unhealthy diet results in high levels or visceral fat, epicardial fat, and hepatic fat, which are also primary indicators of NAFLD.
Fat accumulation in the liver happens due to increased lipid synthesis when compared to lipid disposal. This can be due to increased lipid consumption, increased hepatic de-novo lipogenesis (DNL), decreased fatty acid oxidation, and changes in the ability of hepatocytes to process very low-density lipoproteins (VLDLs). When these metabolic abnormalities arise, this allows the triglyceride rich lipoproteins in the liver to activate toll-like receptors (TLRs) 2 and 4, which activates the NOD-like receptor family, pyrin domain-containing protein 3 (NLRP3). This then increases the presence of proinflammatory cytokines, which can increase the risk of cirrhosis as well as cardiovascular disease.50
NAFLD is one of the most common forms of liver disease, and it affects 25% of people around the world.1 As of 2023, approximately 100 million people suffer from NAFLD in the US, which is more than twice the number it was in the early 2000s.2 This number is predicted to continue to increase, and the percentage of people with NAFLD is projected to be 55.7% in 2040.3 Additionally, NAFLD expenditures are set to increase exponentially in the next 20 years, with an average healthcare spending per capita at $11000 per year in 2010.4 This corresponds to an economic burden of $108 billion in the US due to medical costs alone, as well as an additional $188 billion in societal costs.4
People are more likely to develop NAFLD as they age, and the prevalence of NAFLD in people over the age of 50 is 44.7% as of 2020, which is about 4% more than in people under 50 years old. As much of the developed world is experiencing an aging population, this means that the overall number of people who suffer from NAFLD will continue to increase in the next 20 years.3 Additionally, NAFLD’s economic impacts extend far beyond liver complications, as a significant amount of deaths due to NAFLD occur due to cardiovascular disease.5 Figure 4 depicts a breakdown of the causes of death in people with NAFLD. Figure 4A shows that a majority of people with NAFLD die from cardiovascular disease, and this is followed by cancers of other organs in the body. Liver-related diseases do not tend to be the primary cause of death in people with NAFLD.5 Figure 4B shows the distribution of cancer-related deaths in people with NAFLD. Predictably, the majority of people who die of cancer induced by NAFLD experience tumors in the liver. The next most common form of cancer due to NAFLD is in the lungs.6
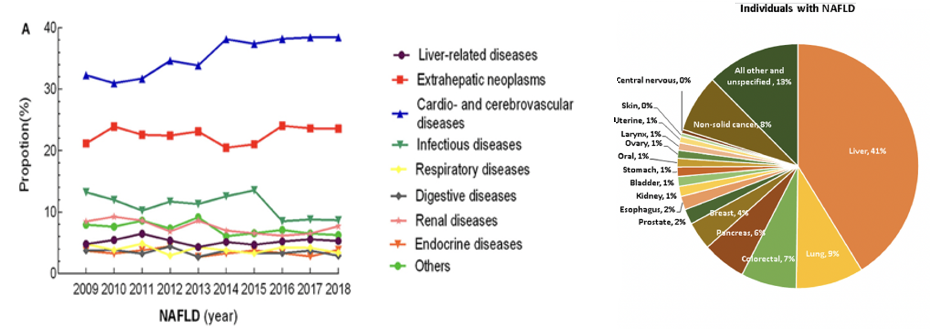
Figure 4 Statistics for NAFLD deaths in the US.
(A) A time-plot depicting the causes of death in people who suffered from NAFLD.6 Cardiovascular diseases make up a significant amount of the deaths, as well as cancerous growths outside of the liver.
(B) A breakdown of the cancer-related deaths in people with NAFLD.5
Liver cancer makes up the most cancer-related deaths, followed by lung and colorectal cancer.
There are very few treatments for NAFLD, and there are no FDA-approved drugs for the treatment or prevention of NAFLD. However, the market for NAFLD treatment in the future can be divided into that of extracorporeal devices and pharmacological drugs, as these are the two avenues that biotechnology companies have conducted research in for the disease. Currently, the best option for people who suffer from advanced NAFLD is a liver transplant.1 This presents a need for products that can treat NAFLD, as over 17,000 patients are left on the waiting list for a liver transplant every year.7 The only other potentially viable treatment for NAFLD is the use of extracorporeal liver support devices, and the vast majority of them are awaiting clinical trials. Albumin dialysis is the most common form of extracorporeal liver support, but some companies are in the process of developing a bioartificial liver for commercial use. Among these companies are Cerce Biomedical Inc., Vitagen Inc., Teraklin, Excorp Medical Inc., and Algenix Inc.8
The liver transplant market has steadily increased in value in the 21st century, and as of 2022, it was valued at $1.5 billion worldwide. Projections for the liver transplant market have valuations at $2.52 billion worldwide. Figure 5 shows how the liver transplant market is projected to grow from 2023 to 2030. While every region in the world is expected to have a marked increase in liver transplants, the Asia-Pacific region is expected to increase the most due to rapid developments in its medical infrastructure.9
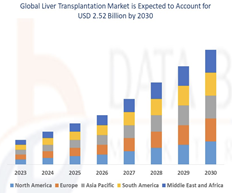
Figure 5 Projected liver transplantation market by region from 2023-2030.9
The compound annual growth rate (CAGR) for the liver transplantation market is roughly 6.70%, and a primary source of that growth comes from the Asia-Pacific region. This is due to improvements in medical infrastructure in that region of the world. By 2030, the liver transplant market is expected to be valued at $2.52 billion worldwide.9
While there are no treatments commercially available for NAFLD, there are a number of drugs undergoing Phase 2 and 3 clinical trials. Key contributors to the NAFLD drug market include, but are not limited to, Gilead, Takeda, Genfit, Intercept Pharmaceuticals, Galmed Pharmaceuticals, and Tocris Bioscience. While these companies have drugs that are in the pipeline for the treatment of NAFLD, nearly every drug that has been tested in Phase 3 trials has failed to prove their viability over a placebo, which has stalled the development of the drug market for NAFLD.10
There are two primary methods for extracorporeal liver support in people with NAFLD: artificial and bioartificial livers. An artificial liver is a device that uses nonliving materials, such as proteins and ion channels, to filter blood outside of the body and then return the blood back to the body.15 A bioartificial liver is a device that, similar to an artificial liver, removes blood from the body and runs it through the device for filtration. Unlike an artificial liver, however, a bioartificial liver uses living hepatocytes to filter the blood.7 Figures 6A&6B depict a typical mechanism for both an artificial and a bioartificial liver system. In a bioartificial system, the blood is removed from the body, and the plasma is separated from the cells. The cells are then transported back into the body, and the plasma is pumped through the device, where it is then oxygenated, treated with charcoal to remove oxidants, and then filtered through the hepatocytes to perform additional detoxification. Often, a bioreactor will be used to culture and maintain a population of hepatocytes in the device. After filtration has been completed, the plasma is diverted back into the bloodstream, and the process must be repeated regularly to maintain blood health.11 Artificial livers work in largely the same way, but a mixture of proteins and other compounds will be used to filter the blood instead of a culture of hepatocytes.15
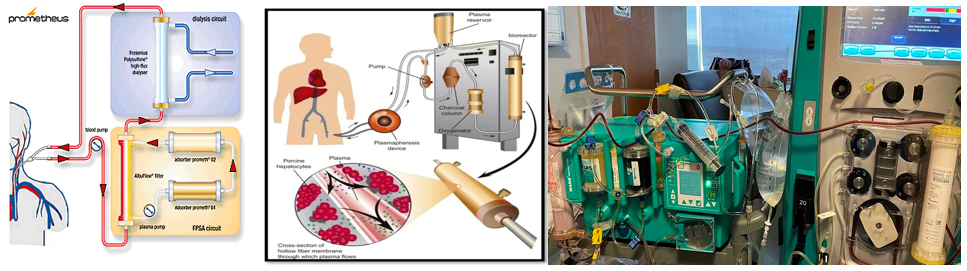
Figure 6 Depictions of artificial and bioartificial livers.
(A) Diagram of the Prometheus liver support system.16 The Prometheus system uses a dialyzer, as well as an adsorber, to filter toxins and excess proteins from blood plasma.
(B) Diagram of a bioartificial liver.11 The blood cells are separated from the plasma and returned to the body. The plasma is pumped through a bioreactor filled with hepatocytes arranged such that exposure to the hepatocytes with the plasma is maximized. The hepatocytes remove toxins from the plasma, and the plasma is returned to the body.
(C) A picture of the MARS liver support system.49
There are five methods that can be used to serve as an artificial liver for people who suffer from NAFLD: molecular adsorbent recirculating system (MARS), single-pass albumin dialysis (SPAD), Prometheus, selective plasma filtration therapy, and hemodiafiltration. The MARS system involves the use of two filtration circuits, both of them containing a solution of albumin. The albumin is circulated through the machine, and the blood plasma is dialyzed via a hemodialysis filter.5 In SPAD, an identical circuit is used when compared to the MARS system, but filtration occurs via countercurrent flow of the plasma and the albumin solution. In the Prometheus liver support system, a machine is preloaded with the patient’s albumin. While the mechanism for filtration is nearly identical to that of MARS, the membrane for the Prometheus system is permeable to albumin, which allows for the patient to recover the albumin that they lost to the machine. In selective plasma filtration therapy, a single-use cartridge containing small tubes for hemodialysis is used to filter out the blood. Some blood plasma is lost to the machine with this method, but it is then replaced with an albumin solution mixed with fresh plasma and electrolytes. In hemodiafiltration, which is primarily used in Japan due to MARS therapy not being approved there, additional waste particles are removed from the body, but hemodiafiltration is different from the other methods because the plasma is infused with coagulation factors and other blood proteins, which is believed to increase the purification of the blood.15
There are a number of bioartificial livers undergoing clinical trials for the treatment of NAFLD and other forms of liver cirrhosis, but they are years away from being suitable for use on patients suffering from the disease. The primary concern with the use of a bioartificial liver is the risk of thrombocytopenia during treatment, and most of the potential products are no more successful at reducing mortalities from NAFLD than standard medical therapies.8 Additionally, the risk of zoonotic disease transmission from porcine hepatocytes is high, and researchers have yet to determine a way to mitigate this risk.3 Table 1 shows a list of the artificial and bioartificial livers that are the furthest along in clinical trials as well as their potential for commercial use.
|
|
Product |
Company/ Institution |
Mechanism |
Development stage |
Primary concern |
|
|
Artificial Livers |
Molecular Adsorbent Recycling System (MARS) |
Teraklin |
Human albumin circuit in anionic exchange column |
Commercialized in 1999 |
Mild thrombocytopenia |
|
|
Single Pass Dialysis (SPAD) |
N/A (Described in Kreymann, Siege et al.20 |
Countercurrent flow through a human albumin channel |
Commercialized in 2005 |
Conflicting evidence regarding effectiveness at treating NAFLD |
||
|
Prometheus |
Fresenius Medical Care |
Similar to MARS, but membrane is permeable to albumin and patient’s own albumin is used |
Commercialized in 1999 |
Mild thrombocytopenia |
||
|
Selective Plasma Filtration Therapy |
Arbios Systems |
Single-use cartridges with porous tubes for plasma filtration |
Commercialized in 2018 |
Little to no effect on blood albumin levels |
||
|
Hemodiafiltration |
Fresnius Medical Care |
Large volume of dialysate is used as substitution fluid |
Commercialized in 2006 |
Expensive, only suitable for acute liver failure |
||
|
Bioartificial Livers |
Extracorporeal Liver Assist Device (ELAD) |
Vitagen |
Cultured human hepatocyte line (C3A) in dialysis cartridge |
Failed Phase 3 trials in 2015 |
Moderate to severe thrombocytopenia |
|
|
HepatAssist 2000 |
Circe Biomedical |
Porcine hepatocytes bound to microcarriers coated in collagen |
Failed shortly after Phase 1 trials in 2005 |
Failed to prove viability over placebo treatment |
||
|
LiverX2000 System |
Algenix, Inc. |
Porcine hepatocytes suspended in colloid solution and injected into intraluminal space |
Failed after Phase 1 trials in 2005 |
Failed to prove viability over placebo treatment |
||
|
|
Modular Extracorporeal Liver System (MELS) |
Charite Virchow Clinic |
Hollow fiber membrane containing patient hepatocytes |
Has not progressed past Phase 1 trials from 2003 |
Failed to prove viability over placebo treatment |
|
Table 1 List of artificial and bioartificial liver products8,11–20
Artificial livers are the only products that have been commercialized for medical use. The primary concerns involving these treatments have either been thrombocytopenia, which is when the platelet levels in the blood are too low, or an inability to prove that they are more effective than a placebo treatment at removing toxins from the blood. Bioartificial livers, on the other hand, have failed to pass Phase 3 trials, and they largely suffer from the same issues as artificial livers. Unlike artificial livers, however, the negative consequences of bioartificial liver treatment are magnified.
There are currently no drugs that have been approved by the FDA for commercial use, but there are many pharmacological products in the pipeline for the treatment of NAFLD. Table 2 shows a list of drugs undergoing Phase 2 and 3 trials for commercial use. A number of metabolic pathways can be targeted by drugs to help reduce the concentration of fatty tissue in and around the liver, and the most common ones are mentioned in Table 2. A commonly targeted pathway for NAFLD treatment is that of lipogenesis, which tends to increase during the early stages of the disease. Reducing activation of this pathway also has the side effect of reducing inflammation in the regions surrounding the liver, which also aids in the mitigation of NAFLD.3 Rencofilstat and MET642 are examples of drugs that reduce lipogenesis in and around the liver, as they target pathways that directly correlate with fat content in the abdomen.23,25 However, targeting metabolic pathways can have multiple unintended side effects, such as an increased risk of cardiac failure and unintended weight gain 34. Another part of the body that therapeutic drugs for NAFLD can target is in the microbiota, as there is a correlation between the prevalence of some species of gut bacteria and the risk of a person developing NAFLD 21. This is yet to be tested, however, and there are no drugs currently that aim to manipulate the microbiome.3
|
Product |
Company |
Mechanism |
Development stage |
Proposed advantages |
Potential disadvantages |
|
Rencofilstat |
Hepion Pharmaceuticals, Inc. |
Cyclophin inhibitor |
Phase 2 |
Cyclophin’s prevalence in multiple metabolic pathways indicates a wide range of therapeutic properties |
Cyclophin’s prevalence can also lead to unintended negative metabolic consequences |
|
Apararenone |
Mitsubishi Tanabe Pharma Corporation |
Mineralocorticoid receptor inhibitor |
Phase 2 |
Antifibrotic effects with heart and renal protection |
Can potentially cause cardiac arrhythmia if administered improperly |
|
MET642 |
Metacrine, Inc. |
Farnesoid X receptor (FXR) agonist |
Phase 2 |
Reduces liver fat content more than other treatments |
Increased metabolism risks unstable weight loss and dizziness |
|
HPG1860 |
Hepagene (Shanghai) Co., Ltd. |
Farnesoid X receptor (FXR) agonist |
Phase 2 |
Reduces liver fat content more than other treatments |
Increased metabolism risks unstable weight loss and dizziness |
|
Miricorilant |
Corcept Therapeutics |
Glucocorticoid receptor antagonist |
Phase 2 |
Reduces alanine transaminase (ALT) and aspartate transaminase (AST) levels |
Potential psychotic effects, as Miricorilant is typically an antipsychotic medication. |
|
Nidufexor |
Novartis Pharmaceuticals |
Farnesoid X receptor (FXR) agonist |
Phase 2 |
Reduces liver fat content more than other treatments |
Increased metabolism risks unstable weight loss and dizziness |
Table 2 List of drugs for the treatment of NAFLD that are undergoing clinical trials21–33
There are no drugs available for the treatment of NAFLD, but a number of them are in Phase 2 trials. Most of them target the Farnesoid X receptor (FXR), which influences metabolic processes and liver fat content, but the drugs have yet to prove that they avoid potential negative metabolic side effects.
NAFLD is a debilitating disease that has significantly increased in prevalence in the 21st century, and to this day, there remain a limited number of legitimate treatments to help patients who suffer from it. While this may be disheartening, there are dozens of medications and devices that are undergoing clinical trials that have the potential to be approved by the FDA within the next decade.35 In the near future, artificial livers will likely be the prevalent form of treatment for NAFLD, alongside exercise and controlled weight loss for a majority of patients, but the NAFLD drug market has the potential to gain traction if any of the drugs listed above can be FDA approved.36
Bioartificial livers were touted to be the future of NAFLD and cirrhosis treatment at the outset of the 21st century, but consistent failures in Phase 2 and 3 clinical trials have made it difficult for the bioartificial liver industry to gain any ground. The main reasons for these failures is that it is difficult to maintain the volume of hepatocytes necessary to filter the entire blood supply of the human body, and the risk of zoonotic disease transmission from xenographic liver cells limits interest in the field.3 Despite this, faith in the viability of bioartificial livers remains high, and it is possible that a bioartificial liver can be commercially available in the near future.39
None.
The authors declare that there are no conflicts of interest.
None.

©2023 Aryan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.