Advances in
eISSN: 2572-8490


Research Article Volume 1 Issue 2
1Department of Chemical Engineering, Brigham Young University, USA
2Department of Psychology, Brigham Young University, USA
3Neuroscience Center, Brigham Young University, USA
Correspondence: Alonzo D Cook, Department of Chemical Engineering, Neuroscience Center, Brigham Young University, USA
Received: October 22, 2016 | Published: December 7, 2016
Citation: Wood RL, Pitt WG, Steffensen SC, et al. A comparison of lysophosphatidylcholine and crush injury in a rat model of sciatic nerve regeneration. Adv Tissue Eng Regen Med Open Access. 2016;1(2):43–50. DOI: 10.15406/atroa.2016.01.00008
This study examined the effect of using lysophosphatidyl choline (LPC) in combination with a nerve crush injury on the healing rate of damaged left sciatic nerves in female rats. The rats were divided randomly into four groups: Control, LPC, Crush or Crush+ LPC. The Control group was the undamaged right sciatic nerve. The other groups were the damaged left sciatic nerve. The healing of the nerves was measured by monitoring gait, electrophysiological parameters: compound muscle action potential (CMAP) amplitudes and nerve conductance velocities (NCV); and morphological parameters: total fascicular area, total myelinated fiber counts, fiber densities, fiber diameters, and g-ratio. Gait and electrophysiological parameters were measured three times a week. Morphological parameters were measured at three weeks and at six weeks. The LPC group was statistically different from the Control group for the first three weeks for the electrophysiological parameters and gait, but was not statistically different from the control at either of the morphological time points. The Crush and Crush+ LPC groups were statistically different from the Control group at week3 for all parameters and only differed from the Control group in the electrophysiological parameter at week 6.The Crush and Crush+ LPC groups did not differ from each other in any parameter at any time point. This study demonstrated that a combination of LPC and Crush did not compound the damage to the nerve, and did not add or subtract healing time to the injury from a Crush.
Keywords: regeneration, lysophosphatidylcholine, sciatic nerve, crush, fiber densitie, action potential, myelination, neurotrophic, degeneration
CMAP, compound muscle action potential; NCV, nerve conductance velocities; LPC, lyso phosphatidyl choline; PNS, peripheral nervous system; DMAE, di methyl amino ethanol; NSA, nonenylsuccinic anhydride; IACUC, institutional animal care and use committee; BBB, basso-beattie-bresnahan; CMAPs, compound muscle action potentials
Every year, hundreds of thousands of patients suffer traumatic injuries to the peripheral nervous system (PNS). PNS injuries generally disrupt the normal functions of sensory and motor neurons through loss of the integrity of axons and Schwann cells.1,2 In severe cases, these injuries permanently destroy motor and sensory functions in limbs. In most cases, healing of the damage to adult peripheral nerves occurs by induction ofcellular responses resembling cellular activity during development.3 However, the damaged peripheral nerves generally regenerate only a fraction of lost motor and sensory function. This study was initiated to establish a crush injury in a rat model and determine the effect of Lysophosphatidylcholine (LPC) on the regeneration of a crushed sciatic nerve.
LPC was chosen because the adminstration of LPC induces acute demyelination,4 which releases the Schwann cells from the axon. Denervating Schwann cells stimulates nerve growth factor (NGF) receptor expression on both the Schwann cell and the axon.5 After injury, axons begin to degenerate distal to the injury site, causing Schwann cells to detach from the axons, which is a necessary step in healing. This loss of contact down-regulates markers of myelination and begins up-regulating developmental markers.6 This causes the Schwann cells to revert to a pre-myelination state. During this pre-myelination state, Schwann cells increase their neurotrophic receptors (e.g. NGFRs) and produce more neurotrophins (e.g. NGF) supporting regeneration.6 After injury, neuronhealing depends on neurotrophin binding to neurotophic receptors.7 After Wallerian degeneration, there is an insignificant increase in neurotrophins around the proximal nerve stump.8 While the nerve can produce all of the necessary factors and receptors to heal itself, it cannot always produce them fast enough or in high enough quantities.9 Wang et al.10 increased a transcription factor from the central nervous system in a damaged sciatic nerve and increased the regeneration of the nerve. This supports Witzel et al.9 conclusion that introducing factors of regeneration is needed for nerve healing. Lee et al.11 suggested increasing the NGF and receptor levels near the lesion site to help increase the rate of axon regeneration. LPC increases both NGF and NGF receptors locally, which is also important in promoting Schwann cell remyelination of the axon.12 Therefore, the hypothesis of this study was that nerve regeneration will be accelerated by the injection of LPC.
In order to elucidate the effects that LPC has on a crushed nerve, the rate of healing of a rat’s sciatic nerve under normal physiological conditions was first established. This included determining the healing rate for a crushed nerve and a nerve that received only LPC without being crushed. The healing rate for a combination of LPC and Crush was also determined. The healing rates were determined by monitoring the rat’s gait, measuring nerve conduction through electromyography, and measuring the fiber density, fiber size, fiber diameter, axon diameter, and g-ratio of each nerve. The regeneration of the left sciatic nerves for each group of rats was compared to a control group, the undamaged right sciatic nerves, at 3 and 6 weeks.
The rats used in this study were Wistar Albino rats of the strain Rattus norvegicus. All rats were 15 weeks old between 250-300g and female. Animals were caged in groups of three until surgery in which they were separated into their own cages. The Lysophosphatidylcholine used in the study was egg derived LPC (Sigma-Aldrich, St. Louis, MO). This was delivered to the nerves at a concentration of 1mg/ml. The solution was prepared by mixing 20mg of LPC with 10mL of saline. This produced a 2X concentration for LPC, which was then added to a 2X solution of fast green to create the correct LPC concentration (1 mg/ml).
Fast green (Sigma-Aldrich, St. Louis, MO) was prepared by mixing 20mg into 10mL of distilled water. This provided the needed 2X concentration which would allow the solution to retain its color when added to the LPC solution. The fast green provided a color to the solution for visual verification during injection. The phosphate buffer used for preserving the nerves was made by dissolving 0.18grams of monobasic sodium phosphate hydrate (NaH2PO4•H20), 2.32grams of dibasic sodium phosphate heptahydrate (Na2HPO4•7H20) and 0.5grams of sodium chloride (NaCl) (Fisher Scientific, Pittsburg, PA) in 100mL of distilled water.
Karnovsky’s fixative was prepared in two steps. The first step was to make a 4.67% paraformaldehyde solution and then make the fixative. For the paraformaldehyde solution, 3.5grams of paraformaldehyde were dissolved in 75mL of distilled water in a 100mL beaker with a magnetic stir bar. The solution was heated to 60-65˚C and stirred at 800 rpm for 30min. Then 1N NaOH was added one drop at a time until the solution became clear (about 7-10 drops). The solution was removed from the heat and allowed to cool, while continuing to stir. Then 0.0001 M HCL, at pH 4, was added one drop at a time until the solution was at pH 8. For the fixative, 10mL of a 10% aqueous glutaraldehyde solution were added in a second 100mL beaker. Then 17.2mL of the paraformaldehyde made in step one were added. Then 12.8mL of a phosphate buffer were added. All of the components were stirred together to result in 40mL of Karnovsky’s fixative.
The Spurr’s resin used for embedding the dehydrated nerves was made by mixing 10g of ERL 4221, 7g of diglycidyl ether of poly(propylene glycol) (DER 736), 26g of nonenylsuccinic anhydride modified (NSA), and 0.4 g of dimethylaminoethanol (DMAE)(Ted Pella Inc., Redding, CA) in a 50mL beaker. All of the components were stirred together, and produced 40 mL of Spurr’s resin.
This study contained five main procedures: gait analysis, electromyography, surgery, nerve preparation and nerve analysis. These procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Brigham Young University.
Gait analysis
The Basso-Beattie-Bresnahan (BBB) scale ranges from 0-21.13,14 and was used to characterize rat gait. The score tracks recovery and categorizes combinations of rat joint movement, hind limb movements, stepping, forelimb and hind limb coordination, trunk position and stability, paw placement and tail position. A score of 0 is indicative of no observable movement of the limb. A score of 21 indicates full movement with “Consistent plantar stepping and coordinated gait, consistent toe clearance; predominant paw position is parallel throughout stance; consistent trunk stability; tail consistently up”.13 Each rat was assessed one day prior to surgery using the BBB scale and three times a week after surgery for the duration of the rat’s experimental process, either three or six weeks.
Electromyography
Nerve conductance through the sciatic nerve fibers was examined under general anesthesia with two percent isoflurane. For transdermal electromyography, stainless steel needles were inserted close to the left sciatic nerve to stimulate the nerve at three locations: ankle, knee, and hip. At each location the needle close to the nerve acted as the cathode, and a remote subcutaneous needle as the anode. A recording needle was placed through the plantar muscles (the sole of the foot) and a reference needle was placed subcutaneously in the heel (Figure 1). Supramaximal stimulations evoked compound muscle action potentials (CMAPs) that were amplified and displayed using a National Instruments multi-function data acquisition module and Lab VIEW software. The difference in base line and the negative peak of CMAPs defined the signal amplitude. Motor nerve conduction velocity (NCV) was measured as the distance between the cathode needles divided by the difference between the latency of two recordings. The NCV was measured between both the ankle and the knee, and the knee and the hip.
The rats in each group were assessed one day prior to surgery to establish pre-surgery amplitudes and velocities. Post-surgery, the rats received electromyography three days a week for the duration of the rat’s experimental process, either three or six weeks. The first post-surgery electromyography was performed within 24 hours of the surgery.
Surgery
Three groups of rats received surgery in which their left sciatic nerves were exposed. The Crush group (N=14) had their sciatic nerves crushed, the LPC group (N=17) received an intraneural injection of LPC, and the Crush+ LPC group (N=17) had their sciatic nerves crushed followed by an intraneural injection of LPC. The Control group, the undamaged right sciatic nerves, provided comparison to establish normality and reduce variability in the results.
Each surgery consisted of the following three protocols: pre-surgery, surgery, and post-surgery. The pre-surgery protocol consisted of the following procedure: twenty-four hours before surgery, the animals were pre-medicated with carprofen (Bio-Serv, Flemington, NJ), an NSAID pain reliever in the form of a chewable tablet. Surgical equipment was sterilized by autoclaving, and placed on the operating table. The operating table and all equipment that could not be autoclaved, such as the microscope and the manipulator, were sterilized with 70% ethanol (Decon Laboratories Inc., King of Prussia, PA) and chlorhexidine (Vet One, Boise, ID). Materials, such as gloves and gauze, were purchased pre-sterilized. Masks and gloves were worn by the surgeon and assistant for the duration of the surgery to maintain the sterile field. Once the sterile field was established, the rat was brought in and anesthetized for the surgery. The rats were anesthetized through the respiration of gaseous isoflurane at three percent for approximately five minutes, or until proper analgesia and sedation was confirmed by applying pressure to the left foot and receiving no response from the rat. The rats then received an injection of the opioid analgesic buprenorphine (Sigma-Aldrich, St. Louis, MO) into the peritoneal cavity. The left leg was then shaved and the rat was placed on the operating table. When moved to the table, anesthesia was monitored so that the rat breathed once every three to four seconds. The shaved portion of the leg was treated with three alternating rubs of 70% ethanol and betadine (Dynarex, Orangeburg, NY), and a sterilized drape was placed over the rat with an opening for the cleaned region on the leg. The rat’s body temperature was monitored by a rectal thermometer and maintained at 37.4˚C by a heating pad driven by a feedback-regulated controller. A towel was placed between the rat and the heating pad to minimize the chance of burning.
The surgery protocol consisted of two procedures, a crush and an injection procedure. For both procedures, a 1cm incision was made starting at the hip and moving towards the knee, and the gluteus superficial is, biceps femoris, and vastus lateralis muscles were separated to expose the sciatic nerve. Once the muscles were separated, a glass hook was used to separate the nerve from the surrounding fat and connective tissue, and provide a reference point for both the crush location and injection sites. For crush injuries, the injury site was measured 0.5cm proximal to the trifurcation into the tibial, sural, and common peroneal branches and the glass hook was placed on the distal side of the measurement. For LPC injections, the injury site was measured 0.5cm proximal to the bifurcation into the tibial and common peroneal branches and the glass hook was placed on the proximal side of the measurement. Once the hook was in place, the surgeon followed the crush and/or injection procedure.
For the crush procedure, forceps were used to crush the nerve. This was done by placing the forceps proximal to the glass hook with the tips approximately 3mm past the edge of the nerve. A force was then applied for 20seconds, released, and then reapplied for 10 additional seconds. The rat’s leg was observed during the crushing procedure to ensure that there was no movement during the last 10seconds of the crush. If movement was detected, an additional 10seconds were added. The force applied to the rats was not measured, since the force was applied by hand, but each surgeon applied a maximum force. The nerve was also verified visually to ensure that the nerve had become less opaque and more transparent.15 Crush surgeries were confirmed if the CMAP amplitudes were equal to or less than 0.2 mV in the first post-surgery electromyography recording.
The LPC injection was performed 3mm proximal to the location of a crush and the fluid was injected towards the distal side. For the injections, a surgical microscope and syringe manipulator were used to insert a 10µm tipped, glass needle into the nerve, parallel to the direction of the nerve fibers. A total of 15µL of fluid was injected into the nerve through a left, center, and right side injection. The solution contained fast green for verification of a correct needle placement and delivery. The injection was verified by a green stripe running down the nerve.
After the crush and/or LPC procedure, the nerve was released from the glass hook and moved back into place with the hook. The incision site was then closed using surgical staples and Neosporin® containing a topical analgesic. The rats were then taken off anesthesia and monitored for 20minutes after waking up. Rats were then monitored every 12hours for the next 48hours and then daily after the first 48hours for the duration of the study. Pain level was monitored according to an institutional veterinarian-approved protocol which divided pain into four categories: weight, clinical signs, appearance, and behavior. In each category the rats received a score ranging from 0-3 with 0 equal to no observed pain or distress, and 3 equal to severe pain or distress. If the rats received a 3 in any category or a combined total greater than 7, the rats were euthanized. If the rats received a 2 in the weight category (10-15% loss of weight in 1 day while still eating) or the score totaled more than 4, buprenorphine was administered to the rat.
Nerve preparation
Following the 3-week or 6-week time period, animals were euthanized using 5% isoflurane for 30min. Euthanasia required 30min because the rats became accustomed to isoflurane. The sciatic nerves from the left and right leg were dissected free and a 1cm section was cut out from the point of the trifurcation moving proximal. The nerves were post-fixed in Karnovsky’s fixative (2% paraformaldehyde/4% glutaraldehyde solution in a phosphate buffer) overnight at 4˚C.
Nerve segments were rinsed at room temperature in phosphate buffer for 1 hour and placed in 2% osmium tetroxide in 0.06M sodium cacodylate buffer for 2 hours. A cacodylate buffer was used because the osmium reacted slightly with the phosphate buffer. The nerves were then washed in distilled water for 1 hour and placed in 5% uranyl acetate in distilled water overnight at 4°C in complete darkness. Nerve segments were then dehydrated through a graded acetone series (10%, 30%, 50%, 70%, 90%, 95%, 100%), cleared through a graded resin series (33%, 66%, 100%), and embedded in Spurr’s Resin. Sections were cut at 1µm using an RMC MTX microtome (Boeckeler Instruments, Tucson, Arizona), placed on Super frost Plus glass slides (Stat Lab, McKinney, TX), and stained with toluidine blue (Sigma-Aldrich, St. Louis, MO). Sections were cover slipped using permount (Electron Microscopy Sciences, Hatfield, PA).
Nerve analysis
For the analysis of the nerve, the distal segment of the nerve was cut off at 3 mm from the trifurcation and sectioned moving distally. This provided an ideal area for analyzing the damage and growth of the nerve due to the treatments. Nerve section images were obtained using a Pentax K100 camera (Ricoh, Malvern, PA) attached to a Zeiss Axiovert 135 microscope (Zeiss, Thornwood, NY) and analyzed using Image J (U. S. National Institutes of Health, Bethesda, MD) software. Whole nerve images were obtained at 400x final magnification. For whole nerve imaging, serial overlapping images were taken over the entire area of interest by manually adjusting the stage area. Mosaic images were formed by reconstructing the images in Adobe Photoshop CS (Figure 2) from which morphometric parameters were derived. The morphometric parameters were calculated using a semi-automated process in Image J. Calculated morphometric parameters were total fascicular area, total myelinated fiber counts, fiber densities, mean fiber diameters, and g-ratio. A fiber consisted of the axon and myelin together.
For the semi-automated process, the brightness and contrast were manually adjusted to the edges of the histogram to better represent the range of image values on the scale. The images were then converted to an 8-bit grey scale image. The threshold value was also manually adjusted to include all parts of each axon. This then created a black and white image in which all values below the threshold value were white and all values above were black. Myelin features were usually darker than the rest of the image, so the background was under the threshold value. The image was then analyzed using Image J’s Analyze Particles module. This provided a defined area range and circularity range to be analyzed. The area range was determined by measuring the smallest and largest axon diameters and calculating their areas. As most axons were fairly circular, the circularity range was chosen to be 0.5-1. An image mask was created with only the particles that fit inside both the area and circularity ranges. Non-axonal particles that were created in the mask were removed by hand by overlaying the original image with 50% opacity and filling in the non-axon regions.16
Using the overlay, the axons that were not detected in the particle analysis could be hand selected on the threshold image. A second mask was created which contained only the selected axons. The two mask images were then merged, with the resulting image containing all of the axons in the nerve (Figure 3). The particle analysis was repeated with the area range set to 0-10,000µm and the circularity from 0-1. This allowed the program to identify all the axons and the results provided the total number of axons, the area and perimeter of each axon, and the diameter at the widest and narrowest part of the axon.
The process for detecting fibers was very similar. In order for the program to identify whole fibers, the resulting axon image from the first part was added to the threshold image, which filled in the axon center in each fiber. Image J’s Analyze Particles module was then run again, using the values for the smallest and largest fiber for the area range and choosing 0.5-1 for circularity. The original image was overlaid to determine which fibers were not analyzed and which areas were not actual fibers. The undetected fibers were added in the same fashion as the undetected axons and the fibers were re-analyzed. The results produced a similar analysis as before, only this time for the fibers (axon+myelin).
From the results of analyzing the fibers and the axons, the total number of myelinated axons and the total number of axons were provided. The g-ratio was determined by first subtracting the axon area from the fiber area to obtain a myelin area and then dividing the myelin area by the axon area. Fiber densities were calculated by dividing the myelinated fiber count by the total fascicular area. The total fascicular area for each nerve was calculated by manually tracing the inner edge of the perineurium for all fascicles and then finding the area of each selection using Image J. Figures 2 & 3 show the process of determining the morphology of the nerve.
Statistics
Total fascicle areas, myelinated fiber counts, fiber densities, fiber packing, mean g-ratio values, NCV and conduction amplitudes were compared between all the groups for both 3-week and 6-weektime points using a one-way ANOVA with a Tukey post-hoc test. Fiber diameter distributions were compared using a Wilcox on signed-rank test. Outliers in data sets were identified using inter quartile criteria prior to statistical tests (outliers were above or below Q3/Q1+/-1.5* IQR respectively). P-values below 0.05 were considered significant. All data are represented as the mean±95% confidence intervals.
To establish the crush injury model and to determine the effects of the LPC on a crush injury, 54 nerves were studied. The number of nerves analyzed were: Control (n=6, two right sciatic nerves from each group), LPC (n=17), Crush (n=14), and Crush+LPC (n=17).
Gait
Experimental results of the gait measurements are shown in Figure 4 beginning with the day before surgery, week 0, through week 6. The LPC, Crush and Crush+LPC groups significantly deviated from the Control group during week 1. The rats that had their sciatic nerves crushed lost nearly all use of the left leg from the knee down after surgery, while the LPC rats only had a decrease in foot usage. The decrease in mobility of the Crush and Crush+LPC group was sustained throughout the first four weeks being significantly different for the first three. The LPC group returned to normal by the fourth week. All of the crushed rats returned to full gait by week 6.

Electrophysiology
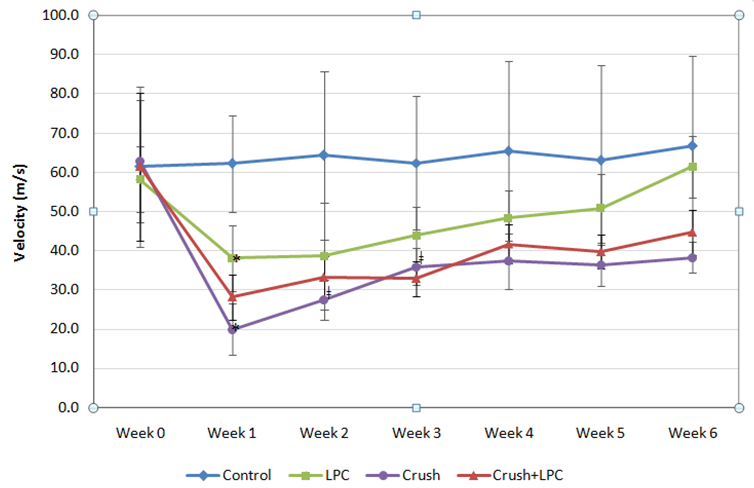
Electrophysiology measurements consisted of CMAP amplitudes and NCV values. The CMAP amplitudes are displayed in Figures 5 & 6. The NCV values are displayed in Figure 7. There was a small difference in the baseline values (Week 0) for each of the groups, but all were statistically similar for both the CMAP amplitudes and NCVs.


Following surgery, an absence of CMAP amplitudes was observed in the Crush and Crush+LPC groups. The crushed nerves slowly increased in CMAP amplitude over the 6-week period. The Crush and Crush+LPC groups only recovered to 16% of the baseline value after week 6 and were statistically different from all other groups every week. The LPC group sustained a decrease in CMAP amplitudes following surgery and recovered by week 4. The LPC group statistically differed from all other groups through week 3. The Control group maintained its CMAP amplitudes across the weeks and were never statistically different one week from another.
The NCV measurements followed a similar pattern as the CMAP amplitudes for the LPC, Crush and Crush+ LPC groups. The Crush and Crush+LPC groups had a significantly different NCV for all six weeks from the Control group, and only statistically differed from LPC at week 1. The LPC group NCVs were significantly different than the Control group for the first three weeks. The Control group maintained its NCV values across all six weeks and never differed one week from another.
Morphology
Regenerated nerve fiber profiles were examined using light microscopy at three and six weeks after damage to determine the healing of damaged nerves and the Schwann cell’s ability to remyelinate axons. Table 1 compares the total fascicular area, total myelinated fiber counts, fiber densities, fiber packing and g-ratio of the control groups to the damaged groups after three weeks of healing. The Crush and Crush+ LPC groups were significantly different than the Control group in the fiber count, fiber packing and mean g-ratio. The diameters of the nerve fibers were measured for each group (Figure 8). All nerve fibers ranged between 0 and 20µm for each group. The distribution of the size of the healing nerve fibers in the distal nerve stump of the Crush group varied slightly from the rest of the groups by having a higher percentage of smaller diameter fibers. None of the groups were statistically different from one another in fiber diameters.
Groups |
Fascicular area (mm2) |
Fiber count |
Fiber density (fiber/mm2) |
Fiber packing (%) |
Mean g-ratio |
Crush |
0.535±0.105 |
4076±1164 |
9416±900 |
11±3 |
0.53±0.12 |
LPC |
0.756±0.155 |
7143±1174 |
9551±1013 |
12±3 |
0.53±0.18 |
Crush+ LPC |
0.604±0.085 |
6177±1479 |
10245±1891 |
12±3 |
0.52±0.16 |
Control |
0.581±0.141 |
6894±1134 |
13364±3513 |
15±3 |
0.56±0.14 |
Table 1 6-week morphology results
Table 1 shows the total fascicular area, total myelinated fiber counts, fiber densities, fiber packing and g-ratio of the control groups to the damaged groups after six weeks of healing. There were no significantly different groups at week 6. Measurements of the nerve fiber diameters showed that the Crush group fibers had healed by week 6 as observed in Figure 9.
This study established the extent of regeneration that occurs after crushing the sciatic nerve and/or administering LPC. In this section of the paper we discuss the benefits of the study, the rationale for the original hypothesis for this work, alternative explanations based on the observations, and recommendations for future work.
The main benefit of conducting this study was that a baseline of healing rate measurements was established in a rat model with LPC and Crush injuries. Gait and morphological parameters were used to establish the healing rate. The morphological parameters: mean g-ratio, total myelinated fiber count, and fiber diameter parameters are characteristic of the extent of Schwann cell damage and healing. Since the morphological parameters were only measured at week 3 and week 6, the data only show whether or not the Schwann cells have healed from the damage. These parameters show that the Schwann cells in the Crush and Crush+LPC groups were still damaged at week 3 but had healed by week 6. The Schwann cells in the LPC group had already started healing by week 3 and had comparable parameters to the Control group.
The electrophysiological parameters provided the rate of motor neuron healing, and suggest that as long as there are a few fibers or axons connected, the nerve can conduct and function regardless of the number of neurons that have healed. This would explain why the rats with crushed sciatic nerves recovered their ability to walk normally by 6 weeks even though the CMAPs portrayed that not all of the motor neurons were healed. Alternatively, the CMAP measurements may not be as reliable as anticipated, because the histological parameters indicated that the nerve should be more healed than the CMAP amplitudes demonstrated (Table 2) (Figure 8).
Groups |
Fascicular area (mm2) |
Fiber count |
Fiber density (fiber/mm2) |
Fiber packing (%) |
Mean g-ratio |
Crush |
0.492±0.087 |
4657±1646 |
9246±2565 |
8±4 |
0.35±0.11 |
LPC |
0.636±0.105 |
5627±1176 |
8847±2222 |
12±5 |
0.53±0.17 |
Crush+ LPC |
0.640±0.088 |
6452±2438 |
9431±2397 |
13±2 |
0.35±0.07 |
Control |
0.437±0.109 |
6767±1017 |
12880±3678 |
18±4 |
0.56±0.18 |
Table 2 3-week morphology results
We recognize that other controls could also be performed, such as a saline-only control, which would primarily test the extent of damage from the needle and fluid injected into the nerve. According to Dyck et al.17 there is some damage done to the nerve fibers from the needle when injecting LPC into the nerve bundle. However, they found that the needle caused very minimal damage, and that when injecting LPC the damage derives primarily from the LPC unless injected by hand. Therefore in this study, we injected LPC with a micromanipulator18 to reduce damage by the needle. We desired to know how injecting LPC into a crushed nerve affected the structure and function of the nerve, including all physical and chemical damage resulting from the injection. Therefore, a saline-injection control was not included, because we were mainly interested in the difference between the healing of each of the three groups: LPC, Crush, and Crush+ PC compared to the non-damaged Control.
The original hypothesis of this work was that LPC would speed up the regeneration of crushed nerves. This hypothesis was based on the knowledge that LPC causes Schwann cells to undergo demyelination.17 It was anticipated that this early demyelination would help speed up the degeneration process, the removal of Schwann cells from the axon, and NGF receptor up regulation, and allow the nerve to begin the regeneration process sooner. Using age-matched experimental groups, less damage was measured from just injecting the nerve with LPC vs. crushing a nerve, but no difference was measured when a crushed nerve also received an LPC injection, contrary to the original hypothesis.
We anticipated that LPC would help the regeneration process due to its ability to interact with Schwann cells. LPC locally demyelinates the nerve but the nerve does not undergo Wallerian degeneration, as occurs after a crush injury. The Schwann cells can still cause the NGF receptors to up regulate and send signaling molecules.18Macrophages are recruited to the site of injury where the myelin is damaged and needs to be cleared away. By combining this very similar process to that of the Wallerian degeneration process, we predicted that the clean-up stage could be achieved quicker and thus the combination of LPC with Wallerian degeneration (crush) would regenerate faster. However, the results show that this is not the case.
Our understanding of the mechanisms in the crush model is that crushed nerves undergo Wallerian degeneration, which is a combination of Schwann cell activity and macrophage recruitment. Schwann cells begin by demyelinating, signaling the macrophages, and begin the initial phagocytosis of the myelin.19 By the second day after injury, the macrophages have arrived from the blood stream and take over the phagocytosis of the myelin.20 Macrophages perform most of the work. Without macrophages, the myelin does not get completely phagocytosed and degeneration moves very slowly.21 Inhibition of complement type 3 receptor in macrophages prevents recruitment to the injury site.22 This is possibly the signaling molecule released by the Schwann cells, which diminishes overtime. Macrophages that arrivelater at the injury site do not begin phagocytosis of the myelin, which is believed to be a result of the diminished signal.21
Schwann cells also up regulate NGF receptors during demyelination. The NGF receptors peak between 5-8 days.23 These receptors may also play a role in initiating the signaling between Schwann cells to form Bands of Bunger for regeneration.23 After the debris has been cleared away, the growth cone of the nerve follows these Bands of Bunger to its distal stump, which marks the regeneration process.
We postulate that there may be four reasons why the expected outcome was not achieved. First, LPC may cause a different signaling molecule to be used than the one used in Wallerian degeneration and thus the body is confused as to what damage has been done. Second, LPC may be increasing the area of damage due to more Schwann cells demyelinating than necessary and thus more cleanup has to happen which slows the process down even if it was able to recruit macrophages faster. Third, the early demyelination of an excessive amount of Schwann cells may be preventing macrophage recruitment to the correct site. Fourth, due to the increase of Schwann cell demyelination, more growth factors are needed than the body would normally produce under normal healing conditions.
Further study is needed to determine if any of the proposed claims are correct. These studies could include a study of the first 10 days after LPC injection, looking at macrophage recruitment, signaling molecules and total extent of the damage area compared to the area of damage in a crush. Future work could also examine the effects of higher concentrations of LPC so that the effect of LPC lasts for more than 3 weeks. Also, a study could be done which examines the effect of exogenous neurotrophins, such as NGF, in this model. With normal healing conditions established by this model, the effectiveness of adding NGF to a Crush+ LPC injury could be determined over a 6 week period. An additional measurement to include in future studies is taking muscle weights at each time point. This would provide an additional measurement for the healing of the motor neurons. It would also help determine if there is any muscle atrophy occurring between the three and six week time points.
This study demonstrated that LPC partially damages the sciatic nerve but does not increase or decrease the amount of damage to a sciatic nerve that has been crushed. The Crush+LPC group was statistically different from the Control and LPC groups for the first three weeks in all parameters and was only statistically different from the groups at the six week time point in CMAP amplitude. The Crush+LPC group was not statistically different from the Crush group at any time point in any parameter. With the findings that the electrophysiological parameters differed from the gait and morphological parameters, it was concluded that using just two morphological time points was insufficient to determine a difference in the healing of damaged sciatic nerves in rats. Future studies examining the effect of LPC at more time points, higher LPC doses, and adding NGF are recommended.
Tyler Brown, Michael Chamberlain, Daniel Elliot, Mitchel Faulkner, Michael Gebhard, Sarah Hansen, Taylor Hansen, Jonathan Jacobs, Keaton Karlinsey, Matthew Landeen, Jordan Miller, Adam Millet, Emil Morco, Mark Rigby, Trent Taylor, Austin Thompson, David Walton, and Stephen Wirthlin assisted with surgeries and electrophysiology measurements. Dr. Keiichiro Susukiwas helpful in teaching us how to perform the surgeries and understanding the data. Funding was provided by the Don B. Olsen Mentorship, and Brigham Young University.
The author declares no conflict of interest.

©2016 Wood, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.