Advances in
eISSN: 2373-6402


Mini Review Volume 4 Issue 3
Department of Plant Protection, Selcuk University, Turkey
Correspondence: Kubilay Kurtulus Bastas, Department of Plant Protection, Selcuk University, Campus, Konya/Turkey, Tel +905-326-362-824, Fax 903322410108
Received: May 25, 2016 | Published: August 8, 2016
Citation: Bastas KK. Top bacterium since 250 years: Erwinia amylovora (Burr.) winslow et al. Adv Plants Agric Res. 2016;4(3):279-281. DOI: 10.15406/apar.2016.04.00138
Fire blight disease caused by Erwinia amylovora is one of the most destructive diseases of pome fruits. Over the past 30 years, many virulence factors of E. amylovora and the genes encoding them have been characterized. Implementation of intelligent quarantine protocols is fully dependent on a comprehensive knowledge of strain diversity and the identification of strains to group. Selection of resistant cultivars and rootstocks in breeding and genetic engineering programmes must take account of the range of diversity for pathogenicity in E. amylovora so that resistance will be durable and universally effective. Integration approach is the best strategy for effective management of fire blight in sustainable and organic growing.
Keywords: fire blight, genetic, organic, control, apple, pear
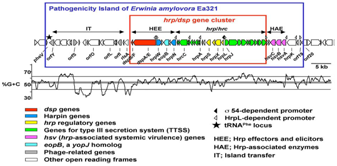
Erwinia amylovora was the first bacterium identified as a plant pathogen. It is the most serious current and long-term threat to fruit cultivation in many parts of the world, as it can infect most plants of the Rosaceae family, both ornamental and economic cultivars, among which apple and pear are important hosts. The typical symptoms on pome fruit trees are the brown to black colour of leaves on affected branches, the production of exudates and the typical “shepherd’s crook” in the shoots.1–3 Depending on the affected plant part the disease produces blossom blight, shoot or twig blight, leaf blight, fruit blight, limb and trunk blight, collar or rootstock blight and cankers (Figure 1). The disease is considered an increasing problem as higher temperatures, breeding of cultivars on susceptible rootstocks and the introduction of susceptible cultivars could enlarge the risk of infection in the near future. The levels of certain nutrients are low in the plant apoplast and the ability to overcome nutrient limitation is essential for rapid population growth and in allowing E. amylovora overcome host defenses. The pathogen produces the hydroxamate siderophore desferrioxamine and iron acquisition systems are important for virulence in E. amylovora. Desferrioxamine was determined as a major factor for protection of E. amylovora against oxidative conditions. In addition, the bacterium cells form biofilms and that biofilm growth may play an important role in host plant colonization. E. amylovora utilizes both a type III secretion system located on a pathogenicity island to deliver effector proteins into host plant cells, and the extracellular polysaccharide (EPS) amylovoran to cause disease. The ams gene cluster, 12 amylovoran encoding genes, involved in the biosynthesis of amylovoran has been characterized. Genes of this island encode proteins that are involved in pathogenicity and virulence (Figure 2).4–8 Effector protein, disease-specific DspA/E, is required for pathogenicity on apple and pear. The dspEF locus is the first-described avirulence locus in E. amylovora. Gene expression analysis of pathogen associated molecular patterns (PAMP) responsive candidate genes revealed Erwinia outer protein 1 (Eop1)’s ability to transcriptionally regulate Malus genes involved in PAMP trigged immunity and Eop1 functions to suppress PAMP-trigged immunity in Malus. Additionally, two harpins, HrpNea and HrpWea, have been characterized in the bacterium. One of the eight plasmids, pEA29, has been described in E. amylovora, and this plasmid plays a role in the expression of EPS. Interestingly, strains of the bacterium isolated from Rubus species are not pathogenic on pear or apple. Another notable report concluded that some cultivars of apple were highly resistant to strain Ea273 but fully susceptible to strain E4001A. Genome sequencing of E. amylovora was reported in late 2004.6–12
The disease is a major threat for the EPPO region, and E. amylovora is one of the most important pests on the EPPO A2 list, A2 pests are locally present in the EPPO region. Because of the great importance of the disease, eradication is generally attempted in newly infested areas. Many species of Crateagus, Pyracantha, Sorbus, Cotoneaster and Stranvaesia as well as ornamental apples, pears and quince are susceptible and these plants may produce inoculum for orchard trees.13 These plants should be watched carefully or be eliminated from the vicinity of commercial apple or pear orchards. If fire blight is present, summer pruning should be done only with disinfested tools and during dry weather. Effective control through pruning requires that cuts are made 30-35 cm (12 to 14 inches) below the visible end of the expanding canker and that between cuts the pruning tools are disinfested with a bleach or alcohol solution to prevent cut-to-cut transmission.14–19 Because E. amylovora overwinters in cankers, as many cankers as practical should be removed during the dormant season. The most fire blight prone cultivars must plant on the highest elevations in the orchard. This will help lower humidity which promotes the bacterium growth. During bloom, it must be avoided from irrigating if possible. If irrigation is required, a drip irrigation system would help keep orchard humidity lower. Soil conditions affect tree growth and tree susceptibility to fire blight. Orchard soils should be maintained at pH 5.5-6.5. The major nutrients, N, P, K, Ca and Mg, and five minor elements, B, Zn, Cu, Mn and Fe, should be applied at rates necessary to maintain a good balance, because imbalance stend to increase the disease severity.20–23
Compatibility with sustainable and organic principles requires growers to first choose varieties that are not susceptible to important diseases in their region. Planting cultivars with either a high level of multi-gene resistance or full immunity would be one potential long-term solution for fire blight control. Genetic engineering expedites the development of disease resistant apple scion varieties as well as rootstocks. Another approach that may be advantageous in developing disease-resistant transgenic plants is to express multiple resistance genes that have different modes of action. In addition to the possible synergistic effects of combining different resistant genes, combining genes with different modes of action may increase the durability of resistance, since bacteria would need to simultaneously overcome two different resistance mechanisms. To date, the genes most extensively used and studied for their effect on fire blight resistance in apple and pear are those coding for cecropins, attacin and lysozymes. The primary biocontrol agents are Pseudomonas fluorescens, Pantoea agglomerans, Bacillus subtilis QST 713, B. amyloliquefaciens, the yeast Aureobasidium pullulans, mycorrhizal fungus Glomus intraradices and synthetic peptides, ESF12, BP76 and BPC194, are also available and organic compliant, providing growers with several options to combine into an integrated fire blight management program.24
Antibiotics, streptomycin and oxytetracycline, are effective and fast-acting, and can be used in concert with disease forecasting and warning models (commonly Maryblyt, Cougarblight, and Billing’s Integrated System) so treatments may only be made when risk of infection is high. However, the use of antibiotics in organic fruit production is incompatible with a system of organic and sustainable agriculture. Early season sprays of Bordeaux mixture have been shown to reduce surface inoculum from cankers. Certain copper products are already used by organic growers during the dormant season to help suppress fire blight bacteria in cankers on the trees. Some promising compounds, which have been registered in some countries, are flumequin, fosetyl-Al and oxolinic acid.25–28 The bioactive products referred to as plant activators/regulators (acibenzolar S methyl, prohexadione-Ca and harpin protein) that induce systemic acquired resistance in plants to many pathogens can be used against fire blight. The disinfection of pear and apple fruits could be accomplished by dipping in 0.1 M citrate buffer (pH 2.5) plus 500p.p.m. DBSA or NaOCl 250p.p.m. plus 200p.p.m. surfactant (Ortho X 77). Control of a wide range of flying and crawling insects including ants, flies, aphids and wasps before bloom help reduce insect-mediated primary inoculation.
During the past quarter century, many genes and gene products have been identified and characterized as being involved in the ability of E. amylovora to cause fire blight in host plants. Recently, experiments to identify host factors necessary to cause the disease were initiated and more comprehensive and extensive experiments will be possible that will likely expand understanding of the virulence factors of the bacterium. Obtaining data, by blocking their normal functions, or by enhancing the activity of host proteins, disease development might be prevented or reduced. These findings may well facilitate the development of improved methods for control of fire blight. There is no single effective chemical including antibiotics or practice that will prevent fire blight infection. Integration approach is the best strategy for effective management of fire blight disease. Successful non-antibiotic fire blight control centers on integrating management practices into a systems approach that is multi-faceted, and combines effective fire blight prevention with fungal control, insect control, apple and pear bloom thinning spray coverage, tree training, chemical control combined with sanitation, pruning, eradication, soil and foliar nutrients, and resistant or tolerant cultivar and root stock selection as listed by Bastas (2015). Introducing a fire blight resistant apple and pear varieties would need to occur over a period of many years and therefore is not a short-term solution to the antibiotic phase-out planned for organic producers. Loop-Mediated Isothermal Amplification technology allows for monitoring and measuring bacterial levels and gives the grower insight into the best spray timing and the level of response required to avoid serious fire blight infection. In the future, this technology will be used starting at first-bloom for near real-time orchard monitoring and population growth of the bacterium.
Climate change affects disease management with regard to timing, preference and efficacy of chemical, physical and biological measures of control and their utilization within IPM strategies. Research is needed in warmer climates on the effects of high daily temperatures (above 30°C) on fire blight epidemiology. Strain identification is also necessary for understanding the dispersal and spread of the pathogen. Ultimately, genetic resistance to the disease will provide the most sustainable alternative but this is likely decades away. The demand for organic apples and pears continues to increase, and growers need well-proven fire blight control approaches to allow them to respond to this demand while minimizing risks from the disease. Planting cultivars with either a high level of multi-gene resistance or full immunity would be one potential long-term solution for fire blight control.
None.
The author declares no conflict of interest.

©2016 Bastas. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.