
Research Article Volume 1 Issue 4
The activity of pathogenesis related proteins in smut resistant and susceptible sugarcane (GT54–9) mutants induced by gamma radiation
Esh AMH,1
Regret for the inconvenience: we are taking measures to prevent fraudulent form submissions by extractors and page crawlers. Please type the correct Captcha word to see email ID.

Guirgis AA,2 El kholi MMA,2 El Absawy EA,2 Nasr MI,2 Hassanien EH3
1Sugar Crops Research Institute, Cairo University, Egypt
2Genetic Engineering and Biotechnology Research Institute, Minufiya University, Egypt
3Faculty of Agriculture, Ain Shams University, Egypt
Correspondence: Esh AMH, Sugar Crops Research Institute, Cairo University, Agricultural Research Center, 9 Cairo university street, Giza sugar crops research institute, Tel +201005506639
Received: August 25, 2014 | Published: October 13, 2014
Citation: Esh AMH, Guirgis AA, El-kholi MMA, et al. The activity of pathogenesis related proteins in smut resistant and susceptible sugarcane (GT54–9) mutants induced by gamma radiation. Adv Plants Agric Res. 2014;1(4):146-156. DOI: 10.15406/apar.2014.01.00024
Download PDF
Abstract
The activity of six pathogenesis related (PR) proteins (polyphenole oxides, phenylalanine ammonia lyase, peroxidase, esterase, chitinase and β 1,3 glucanase) in sugarcane were used to detect the variation between smut susceptible and resistant sugarcane clones generated from the moderately resistant sugarcane cultivar GT 54-9 using gamma radiation. Activity of PR proteins was monitored in healthy and artificially infected plants. A dramatic increase in the tested enzymes (phenylalanine ammonia lyase, peroxidase, esterase and chitinase) was noticed in the resistant infected (RI) plants compared to the susceptible infected (SI) plants and to the control. Generally, the levels of the tested enzymes in the (SNI) plants were lower than those recorded in the (RNI) or the moderately resistant (GT 54-9 cultivar) control plants.
Keywords: Sugarcane smut; Ustilago scitaminea; Pathogenesis related proteins; Polyphenole oxidase; Phenylalanine ammonia lyase; Peroxidase; Esterase; Chitinase; β1,3 Glucanase
Abbreviations
PR: Pathogenesis Related Proteins; PPO: Polyphenol Oxidase; PAL: Phenylalanine Ammonia Lyase; EST: Esterases; POX: Peroxidase; IDOC: % of Increase/Decrease Over Control; RI: Resistant Infected; SI: Susceptible Infected; Syd: Sporisorium Scitamineum; RNI: Resistant Non-Infected
Introduction
Smut is an important economic disease of sugarcane. The causal organism, Ustilago scitaminea Syd.; (Sporisorium scitamineum (Syd.) M. Piepenbr., M. toll & Oberw.) was first reported in Natal in 1877 in South Africa and now smut occurs in most of the sugarcane producing areas of the world [1]. The disease aggressively affects plant growth and cane yield [2].
Plants producing a number of compounds and proteins in response to pathogen infection. These compounds and proteins are believed to have a high importance in protecting them from the deleterious effects of the pathogen. These include the accumulation of antimicrobial compounds [3-5] and the physical strengthening of plant cell walls through increased production of hydroxyproline-rich glycoproteins, lignin and suberin [6-8]. The produced proteins (pathogenesis-related (PR) proteins) are known to be highly resistant to proteolytic degradation [9]. The PR proteins have been classified into 14 families based on the amino acid sequences, serological relationship and/or enzymatic or biological activity [10]. Many PR proteins exhibit direct antifungal activity against a wide range of fungal pathogens [11].
Resistance to smut has been associated with the accumulation of free or conjugated polyamines in sugarcane tissues [12,13]. Several glycoproteins produced in sugarcane tissues during the infection process [2] found affecting the fungal spore germination negatively [14-16]. Some of glycoproteins have the ability to affect the cytoplasmic polarity during spore germination [14] and impede cell polarization by inhibiting the protrusion of the germ tube and spore germination. The inhibition of teliospore germination constitutes a defense mechanism involved in the resistance of sugarcane to smut [15,16].
The aim of this study was to investigate the PR proteins changes in young sugarcane plants and to relate susceptibility or resistance to smut with changes in the levels of PR proteins produced by inoculated smut sporidia.
Materials and Methods
Plant materials
Ten smut resistant sugarcane clones were obtained from (Sugar Crops Research Institute, Giza, Egypt) (GT 54-9, C9/0.5Kr-11, C9/0.5Kr-32, C9/0.5Kr-42, C9/0.5Kr-46, C9/1Kr-3, C9/2Kr-1, C9/3Kr-63, C9/3Kr-69 and C9/3Kr-8) and 10 susceptible clones (C9/2Kr-19, C9/2Kr-4, C9/3Kr-38, C9/3Kr-45, C9/3Kr-47, C9/3Kr-52, C9/3Kr-56, C9/3Kr-70, C9/1Kr-3 and C9/3Kr-79). All clones were selected from a former mutation induced clones by gamma radiation to the moderately resistant sugarcane cultivar GT 54-9.
Sugarcane stalks of each mutant were stripped of all leaves, cut into one bud setts, then given a hot water treatment for 10 min at 52 °C to stimulate growth. The setts were dipped into 300 mg a.i. / L fungicide solution (Benomyl). The setts were cultivated (3 cm depth) in 25 cm diameter pots filled with sand, pitmos and soil (1:1:1) each pot contained 3 setts. The greenhouse temperature was 28 °C ± 2 [17].
After the germinating shoots reach 20 cm long (45 days old), pots of each mutant divided to two groups over three replicates (3 pots each) the plants of the first group inoculated with U. scitaminea and the plants of the second group inoculated with sterile distilled water to serve as a control.
Fungal material
Single haploid plus (+) and minus (-) spores from germinated teliospores of U. scitaminea -isolated from commercial field located in Quena governorate, Egypt were prepared using the method described by [18]. Two isolates of single haploid (+) and (-) yeast-like culture of U. scitaminea grown on GYC medium for 72 h. at 28 °C. After incubation the cultures centrifuged at 8000 rpm for 5 minutes. The supernatant discarded and the collected cells washed 2 times using sterile distilled water and adjusted to reach 2x105 spores per ml using a haemocytometer. Equal volumes of haploid (+) and (-) yeast-like spore suspension were mixed and incubated for 3 h. at 30 °C before inoculation [18]. To reduce surface tension, Tween 20 was added at a rate of 100 ul per 100 ml of spore suspension prior inoculation [17].
Inoculation
The hypodermic injection technique according to [17] was used to inoculate the emerged plants. Plant Shoots were inoculated when they reached 20 cm long using 50 μl of a suspension containing 2×105 sporidia /ml of a 1:1 mixture (plus and minus) of the isolated mating cell types (inoculated plants) or with 50 μl of sterile water (non-inoculated). Inoculation was carried out in the apical portion of the stem through the leaf sheath to ensure its contact with the meristematic region of the stem, which is the specific site of mycelium penetration and development in nature, when airborne dispersed teliospores are deposited on vegetative buds. Inoculum was infected into the stem 3 cm above the first leaf with a visible dewlap. Smut inoculated and non-inoculated plants were sampled at 120 h post-inoculation. Stem segments of 6 cm long (3 cm above and below the point of inoculation) of the inoculated and non-inoculated plants sampled at different time intervals were ground in liquid nitrogen. Total protein in each clone was determined according to [19]. Samples of 1.0 g of the fine powder were extracted for each enzyme system. The percentage of increase /decrease over control (%IDOC) of each enzyme in infected and non-infected plants was calculated using the formula: ((T-C)/C)×100
Where:
C = enzyme level in infected or non infected control GT 54-9 cultivar.
T =enzyme level in infected or non infected clones.
Analysis of Polyphenol Oxidase (PPO) activity
Sugarcane samples were extracted according to the method described by Malik & Singh [20]. The enzyme extract was prepared by suspending 1.0 g sample in 0.1 M sodium phosphate buffer pH 7 (2 ml / g fresh weight), then centrifuged at 6000 rpm for 30 min. under 4 °C, the clear extract was collected, completed to 15 ml volume using phosphate buffer and used as a crude enzyme source. The reaction mixture contained 0.2 ml of crude enzyme source, 1 ml of phosphate buffer pH 7; 1 ml of 10-3 M catechol and completed with distilled water up to 6 ml. The reaction was incubated for 30 min. at 30 °C. One unit of PPO was expressed as the change in absorbance at 420 nm, and expressed as unit’s min−1 g−1 fresh wt. [21].
Analysis of Phenylalanine Ammonia Lyase (PAL) activity
A procedure described by Zucker [22] was used; one gram of sample was suspended in 5 ml of 0.1 M borate buffer (pH 8.8) and 54 mM β- mercaptoethanol. The mix centrifuged at 10,000 rpm at 4 °C for 10 minutes then 1 ml of the supernatant was mixed with 1 ml 30 mM of phenylalanine and 1 ml borate buffer and incubated for one hour at 40 °C for reaction termination, 0.2 ml of 5 N HCl was added then the volume increased up to 4 ml using distilled water. The amount of transcinnamic acid formed in the reaction was measured at 290 nm and calculated according to Saunders & Mc Clure [23] as the change in absorbance of 0 .01 is equivalent to the production of 3 .09 n moles of cinnamic acid. The specific activity of the enzyme was expressed as moles of cinnamic acid produced per hour/gm of the tissue.
Extraction procedure for peroxidases and esterases
For estimation of peroxidases and esterases, one gram of sugarcane sample was suspended in 2 ml of cold freshly prepared 10% polyvinylpyrrolidone in 0.5 M tris HCl buffer (pH 7.2) and the ratio was kept 2:1 (v/w) for buffer and material. The slurry was centrifuged at 14000 rpm for 20 minutes at 4 °C, and the resulted supernatant was used for enzyme assay.
Estimation of Esterases (EST)
One ml of crude enzyme was added to 5 ml of the reaction mixture and kept at 37 °C for 1 h. The reaction mixture was prepared by dissolving 200 mg α-napthyl acetate in 10 ml of 50% acetone and 200 mg Fast Blue RR salt in 90 ml of 0.2 M of phosphate buffer (pH 7.0). The components of the reaction mixture were mixed together and filtered through Whatman No. 1 filter paper in the dark at 4 °C. The activity was measured at 600 nm and expressed as unit’s min−1 g−1 fresh wt [24].
Estimation of peroxidases
The reaction mixture was prepared as previously described by Malik & Singh [20]. The mixture contained, 0.5 ml phosphate buffer pH 7; 0.2 ml enzyme source; 0.3 ml of 0.05 M pyrogallol; 0.1 ml of 1%(v/v) H2O2. The total mixture volume was raised to 3 ml using distilled water. The reaction mixture was incubated at 30 °C for 5 min. Then the reaction stopped by adding 0.5 ml of 5 % (v/v) H2O2 [21]. One unit of peroxidase activity was expressed as the changes in absorbance at 425 nm and expressed as unit’s min−1 g−1 fresh wt.
Determination of Chitinase
One gram of sample was suspended in an extraction buffer consisting of 0.1 M acetate buffer (pH 5.0) containing 0.1% (W/V) each of ascorbic acid and sodium sulphite and 5% PVP. The homogenates were centrifuged at 12,000 rpm at 4 °C for 30 min., and then the supernatant was used for enzyme assay. A mixture of crude enzyme source (1 ml) and suspension of colloidal chitin (1 ml; 0.1% in 50 mM sodium acetate buffer; pH 5) was incubated at 38 °C in a water bath with constant shaking. After 2 hr, the release of N-acetylglucosamine in the reaction mixture was estimated by the method of Reissig et al. [25]. The enzyme activity was determined using N-acetylglucosamine (Sigma) as the standard. Absorbance was measured at 660 nm. One unit of chitinase is defined as the amount of enzyme producing 1 μ mol N-acetylglucosamine / min in 1 ml of reaction mixture under standard assay conditions. Specific activity was expressed as unit’s min−1 g−1 fresh wt.
Determination of ß-1, 3 glucanase
One gram of sample was suspended in an extraction buffer consisting of 0.1 M acetate buffer (pH 5.0) containing 0.1% (W/V) each of ascorbic acid and sodium sulphite and 5% PVP. The homogenates were centrifuged at 12,000 rpm at 4 °C for 30 min., the supernatant was used for enzyme assay [26].
Total activity of ß-1, 3 glucanase was determined by measuring the released reducing sugar from laminarin (Sigma-Aldrich) as a substrate [26]. The assay mixture was consisted of 0.8 ml of 0.1M acetate buffer pH 5.0 containing 1% laminarin and 0. 4 ml of enzyme extract. After 30 minutes incubation 30 °C, the reducing substances were colorimetrically estimated according to [27,28] at 660 nm. The standard curve of glucose was used as reference. Specific activity was expressed as unit’s min−1 g−1 fresh wt.
All the chemicals and reagents used in this work were produced by Sigma Aldrich and Amresco. The colorimetric assays were carried out using Spectronic 601; Milton Roy, Rochester, NY spectrophotometer. Data of the present work were statistically analyzed by analysis of variance using a complete randomize design with 3 replicates according to [29] using SPSS system version 8 [30].
Results and Discussion
The germination of Ustilago scitaminea spores occurs on the internode surface and it is followed by appressoria formation, mainly on the inner scale of young buds and on the bases of emerging leaves [31]. The entry of the germ tube into the bud meristem occurs between 6 and 36 h., after the teliospores are deposited on the surface [32]. After the infection, the fungal hyphae grow throughout the infected plant, but mostly in the parenchyma cells of the lower internodes. In the upper internodes, hyphal growth concludes with the formation of the whip (sori with teliospores). It has been proposed that varied resistance of sugarcane is determined by several morphological features of buds [31]. In this work we used injection as an inoculation method to determine the physiological resistance.
Polyphenoloxidase (PPO) activity
The levels of polyphenoloxidase in the resistant infected (RI) and non-infected (RNI) sugarcane clones (Table 1) were significantly higher than in the susceptible infected (SI) and non-infected (SNI) sugarcane clones. In susceptible clones, it was noticed that the levels of PPO were lower than in the control (GT 54-9 cultivar) in both infected and non-infected treatments while in resistant varieties the levels of PPO were higher in non-infected treatment except the clones C9/3Kr-63 and C9/3Kr-8 and the clones C9/0.5Kr-46, C9/1Kr-3, C9/3Kr-63, C9/3Kr-69 and C9/3Kr-8 in infected treatment. In susceptible clones, the percentage of PPO level reduction compared to that in control (% IDOC) ranged from 9.61 to 41.98% in non infected plants and from 37.99% to 52.31%, while in resistant clones the increase of PPO level in non-infected clones ranged from 0.27% and 26.74% and the reduction was less than 3% in two clones and in infected clones the increase ranged from 2.63 to 5.51% and the reduction ranged from 0.56 to 8.1% (Figure 1).
Resistance |
Clone |
Polyphenoloxidase (Units min−1 g−1 fresh wt.) |
Mean |
Non-Infected |
% IDOC* |
Infected |
% IDOC* |
Susceptible |
C9/2Kr-19 |
22.48 lmn |
↓33.38 |
25.48 lm |
↓48.86 |
24.4231 B |
C9/2Kr-4 |
19.58 n |
↓41.98 |
25.85 l |
↓48.12 |
C9/3Kr-38 |
20.90 mn |
↓38.09 |
25.28 lm |
↓49.26 |
C9/3Kr-45 |
20.93 mn |
↓37.98 |
24.68 lm |
↓50.46 |
C9/3Kr-47 |
22.87 lmn |
↓32.25 |
26.78 kl |
↓46.23 |
C9/3Kr-52 |
22.31 lmn |
↓33.91 |
24.26 lmn |
↓51.31 |
C9/3Kr-56 |
23.78 lmn |
↓29.55 |
26.98 kl |
↓45.85 |
C9/3Kr-70 |
23.50 lmn |
↓30.38 |
23.76 lmn |
↓52.31 |
C9/1Kr-13 |
22.34 lmn |
↓33.82 |
25.24 ijk |
↓49.34 |
C9/3Kr-79 |
30.51 jk |
↓9.61 |
30.89 ijk |
↓37.99 |
|
Mean |
22.9234 |
|
25.9228 |
|
|
Resistant |
C9 Cont. |
33.76 ij |
|
49.83 abcd |
|
43.9238 A |
C9/0.5Kr-11 |
42.55fgh |
↑26.06 |
51.78 abc |
↑3.91 |
C9/0.5Kr-32 |
40.97 h |
↑21.36 |
53.85 a |
↑8.06 |
C9/0.5Kr-42 |
42.79 fgh |
↑26.74 |
51.14 abc |
↑2.63 |
C9/0.5Kr-46 |
41.17 gh |
↑21.96 |
48.95 bcd |
↓1.76 |
C9/1Kr-3 |
33.85 ij |
↑0.27 |
47.50 cde |
↓4.67 |
C9/2Kr-1 |
44.14 efgh |
↑30.74 |
52.58 ab |
↑5.51 |
C9/3Kr-63 |
32.88 ij |
↓2.59 |
47.09 cdef |
↓5.49 |
C9/3Kr-69 |
35.43 i |
↑4.94 |
45.79 defg |
↓8.10 |
C9/3Kr-8 |
32.83 ij |
↓2.74 |
49.55 abcd |
↓0.56 |
|
Mean |
38.0400 |
|
49.8075 |
|
Mean |
34.2631 B |
|
43.1359 A |
|
|
LSD at (0.05) for: |
|
|
|
|
|
Resistance (R): |
6.361 |
|
|
|
|
Infection (I): |
5.724 |
|
|
|
|
Clones (C ): |
5.724 |
|
|
|
|
RxIxC: |
4.217 |
|
|
|
|
Table 1: Specific activity of Polyphenoloxidase (PPO) in healthy and U. scitaminea infected sugarcane resistant and susceptible mutants.
*IDOC: % of Increase/Decrease over control, up arrows (↑) = increase down arrow, (↓) = decrease
*Different letters in means indicate a significant difference
PPO: Polyphenoloxidase
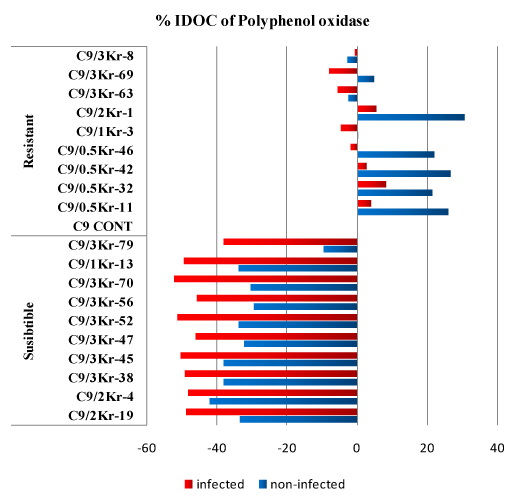
Figure 1: Percentage of Increase/Decrease over control (%IDOC) of polyphenoloxidase (PAL) in healthy and U. scitaminea infected sugarcane resistant and susceptible GT54-9 mutants.
Many workers have reported the increase in the activity of polyphenole oxidase in resistant varieties. Polyphenol enzymes (catecholase and cresolase) have been reported to be responsible for in vivo synthesis and accumulation of phenolic compounds [33,34].
The importance of polyphenoloxidase activity in disease resistance is due to its property to oxidize phenolic compounds to quinines which are often more toxic to microorganisms than the original phenols. It is reasonable to assume that an increased activity of polyphenoloxidase results in higher concentration of toxic products of oxidation and therefore causes greater degree of resistance to infection [35]. Sundar et al. [36] reported that polyphenoloxidase activity is related to resistance against red rot disease in sugarcane.
Phenylalanine Ammonia lyase (PAL) activity
The results demonstrated that the specific activity of Phenylalanine ammonia lyase showed a similar pattern of increase as for the activity of PPO in selected resistant and susceptible clones of sugarcane. In the present study, it was noticed that the PAL activity was significantly higher in infected and non infected resistant clones compared to the susceptible ones (Table 2).
In susceptible clones the percentage of PAL level reduction than in control GT 54-9 cultivar. The % IDOC decreased in susceptible clones and ranged from 37.66 to 55.84% in non-infected plants and from 2.48 to 18.73% in infected plants except for four clones (C9/3Kr-45, C9/3Kr-47, C9/3Kr-52 and C9/3Kr-56) the PAL activity increased by (11.11, 27.69, 16.08 and 0.16% respectively) compared to the control. On the other hand, in resistant non-infected clones the % IDOC decreased compared to the control. The reduction of PAL activity ranged from 8.22 and 22.07%. In resistant non-infected clones PAL activity significantly increased to the maximum 32.0% and a minimum of 22.55% (Figure 2).
Resistance |
Clone |
PAL (moles of transcinnamic acid / h−1 g−1 fresh wt) |
Mean |
Non-Infected |
% IDOC |
Infected |
% IDOC |
Susceptible |
C9/2Kr-19 |
0.0084 lmn |
↓45.67 |
0.0163 gh |
↓18.73 |
0.0140 B |
C9/2Kr-4 |
0.0068 mn |
↓55.84 |
0.0196 de |
↓2.48 |
C9/3Kr-38 |
0.0085 lm |
↓44.80 |
0.0183 def |
↓8.78 |
C9/3Kr-45 |
0.0081 lmn |
↓47.61 |
0.0223 c |
↑11.11 |
C9/3Kr-47 |
0.0069 lmn |
↓54.97 |
0.0257 a |
↑27.69 |
C9/3Kr-52 |
0.0076 lmn |
↓50.43 |
0.0233 bc |
↑16.08 |
C9/3Kr-56 |
0.0073 lmn |
↓52.59 |
0.0201 d |
↑0.16 |
C9/3Kr-70 |
0.0088 l |
↓43.07 |
0.0180 efg |
↓10.28 |
C9/1Kr-13 |
0.0096 n |
↓37.66 |
0.0196 fgh |
↓2.48 |
C9/3Kr-79 |
0.0065 n |
↓57.79 |
0.0172 fgh |
↓14.42 |
|
Mean |
0.0078 |
|
0.0201 |
|
Resistant |
C9 Cont |
0.0154 hi |
- |
0.0201 d |
- |
0.0191 A |
C9/0.5Kr-11 |
0.0123 jk |
↓20.12 |
0.0265 a |
↑32.00 |
C9/0.5Kr-32 |
0.0127 jk |
↓17.74 |
0.0253 a |
↑25.87 |
C9/0.5Kr-42 |
0.0141 ij |
↓8.22 |
0.0265 a |
↑31.84 |
C9/0.5Kr-46 |
0.0131 jk |
↓14.71 |
0.0255 a |
↑27.03 |
C9/1Kr-3 |
0.0126 jk |
↓18.18 |
0.0253 a |
↑25.70 |
C9/2Kr-1 |
0.0129 jk |
↓16.01 |
0.0246 ab |
↑22.55 |
C9/3Kr-63 |
0.0133 jk |
↓13.85 |
0.0261 a |
↑30.01 |
C9/3Kr-69 |
0.0142 ij |
↓7.57 |
0.0246 ab |
↑22.55 |
C9/3Kr-8 |
0.0120 k |
↓22.07 |
0.0255 a |
↑27.03 |
|
Mean |
0.0133 |
|
0.0250 |
|
Mean |
0.0118 B |
|
0.0232 A |
|
|
LSD at (0.05) for: |
|
|
|
|
|
Resistance (R): |
0.00232 |
|
|
|
|
Infection (I): |
0.00562 |
|
|
|
|
Clones (C ): |
0.00232 |
|
|
|
|
RxIxC: |
0.001709 |
|
|
|
|
Table 2: Specific activity of phenyl alanine ammonia layase (PAL) in healthy and Ustilago scitaminea infected sugarcane resistant and susceptible mutants.
*IDOC: % of Increase/Decrease over control, up arrows (↑) = increase down arrow, (↓) = decrease
*Different letters in means indicate a significant difference
PAL: Phenyl Alanine Ammonia Layase
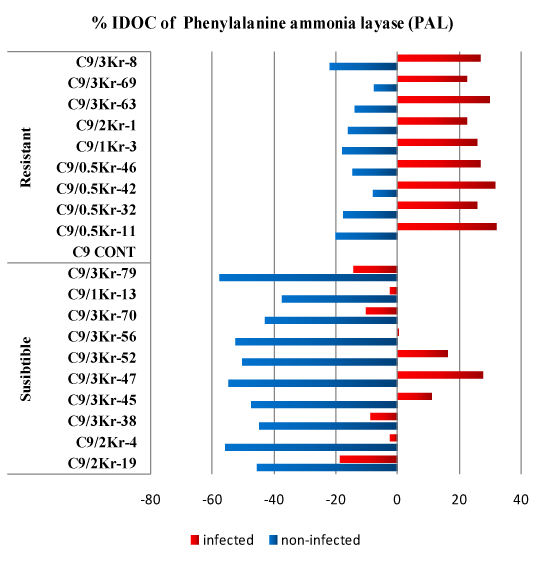
Figure 2: Percentage of Increase/Decrease over control (%IDOC) of Phenylalanine ammonia layase (PAL) in healthy and Ustilago scitaminea infected sugarcane.
Phenylalanine ammonia lyase activity is fundamental to maintain or increase the synthesis of all these phenolics and according to De Armas et al. [37], resistance to smut in sugarcane is associated with the possibility of maintaining high levels of PAL activity. Singh et al. [38] reported higher activity of PAL in red rot resistant cultivars of sugarcane than the susceptible ones. On the other hand, [39-41] reported that the increase of PAL is associated with the increase in lignin synthisis in disease resistant plants.
Crude elicitor prepared from S. scitamineum mycelium induces high phenylalanine ammonia-lyase activity without accumulation of free hydroxyl cinnamic acids and moderately high peroxidases activity, mainly in resistant cultivars [37].
Analysis of Esterase (EST)
The levels of esterases were significantly increased in the (RI) and (RNI) clones compared to the infected and non-infected control, respectively, as well as the (SI) and (SNI) control. The percentage of IDOC in the resistant non-infected clones ranged from 1.69% to 41.13% compared to the non-infected control while in the (RI) clones, the increase ranged from 5.99% to 23.62% compared to (RNI) control. In infected susceptible clones the enzyme level decreased significantly compared to the infected control, the reduction ranged from 2.58% to 20.47% (Table 3).
Kim et al. [42] reported that esterase of Capsicum annum inhibit the appressorium formation of Glomerella cingulata. Zhang & Birch [43] reported a detoxification gene for Albicidin from Pantoea dispersa. The gene encodes an esterase and it abolishes the capacity of Xanthomonas albilineans to release albicidin toxin and incite the symptoms of leaf scald disease in sugarcane. Koretsky [44] suggested the role of esterases in the development of resistance to fusarium infection in soy bean (Figure 3).
Resistance |
Clone |
Esterase healthy (units min−1 g−1 fresh wt) |
Mean |
Non- Infected |
% IDOC |
Infected |
% IDOC |
Susceptible |
C9/2Kr-19 |
0.1868 [ |
↓20.05 |
0.2988 m |
↓13.96 |
0.2568 B |
C9/2Kr-4 |
0.1865 [ |
↓20.19 |
0.2963 n |
↓14.67 |
C9/3Kr-38 |
0.1827 / |
↓21.83 |
0.2885 o |
↓16.92 |
C9/3Kr-45 |
0.2070 y |
↓11.42 |
0.2970 n |
↓14.48 |
C9/3Kr-47 |
0.2342 w |
↑0.19 |
0.3184 k |
↓8.32 |
C9/3Kr-52 |
0.2037 z |
↓12.85 |
0.2873 o |
↓17.26 |
C9/3Kr-56 |
0.2370 v |
↑1.41 |
0.3383 h |
↓2.58 |
C9/3Kr-70 |
0.2298 x |
↓1.65 |
0.3284 i |
↓5.44 |
C9/1Kr-13 |
0.1864 [ |
↓20.23 |
0.2772 p |
↓20.47 |
C9/3Kr-79 |
0.2442 t |
↑4.47 |
0.3077 l |
↓11.41 |
|
Mean |
0.2098 |
|
0.3037 |
|
Resistant |
C9 Cont |
0.2337 w |
|
0.3473 g |
|
0.3333 A |
C9/0.5Kr-11 |
0.2607 s |
↑11.53 |
0.3883 d |
↑11.79 |
C9/0.5Kr-32 |
0.2392 u |
↑2.33 |
0.3783 e |
↑8.93 |
C9/0.5Kr-42 |
0.2735 q |
↑17.03 |
0.3981 c |
↑14.63 |
C9/0.5Kr-46 |
0.2377 uv |
↑1.69 |
0.3880 d |
↑11.71 |
C9/1Kr-3 |
0.2777 p |
↑18.81 |
0.3681 f |
↑5.99 |
C9/2Kr-1 |
0.3222 j |
↑37.85 |
0.4007 b |
↑15.36 |
C9/3Kr-63 |
0.2680 r |
↑14.67 |
0.4293 a |
↑23.62 |
C9/3Kr-69 |
0.3298 i |
↑41.13 |
0.3879 d |
↑11.69 |
C9/3Kr-8 |
0.3090 l |
↑32.22 |
0.4279 a |
↑23.19 |
|
Mean |
0.2751 B |
|
0.3914 A |
|
Mean |
0.2587 |
|
0.3664 |
|
|
LSD at (0.05) for: |
|
|
|
|
|
Resistance (R): |
0.0023 |
|
|
|
|
Infection (I): |
0.0036 |
|
|
|
|
Clones (C ): |
0.0023 |
|
|
|
|
RxIxC: |
0.0017 |
|
|
|
|
Table 3: Specific activity of Esterase (EST) in healthy and U. scitaminea infected sugarcane resistant and susceptible mutants.
*IDOC: % of Increase/Decrease over control, up arrows (↑) = increase down arrow, (↓) = decrease
*Different letters in means indicate a significant difference
EST: Esterase
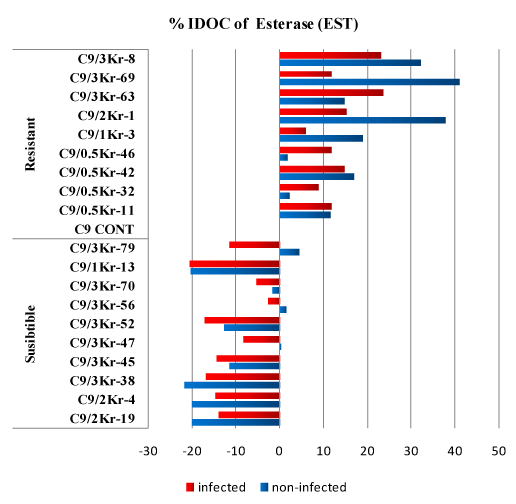
Figure 3: Percentage of Increase/Decrease over control (%IDOC) of Esterase (EST) in healthy and Ustilago scitaminea infected sugarcane resistant and susceptible GT54-9 mutants.
Analysis of Peroxidase (POX)
Data presented in (Table 4) show that the enzyme levels significantly increased in the (RI) and (RNI) control compared to the (SI) and (SNI) clones. In the resistant non-infected control the enzyme level increased 29.96% to 66.07% over the non-infected control. On the other hand the increase of enzyme levels in the (RI) clones ranged from 5.20% to 27.02% over the (RI) control. The enzyme level in the (SI) clones almost decreased 2 folds compared to the infected control. The range of enzyme reduction ranged from 29.73% to 46.43%). The obtained data suggest the presence of an association between resistance and the increase of peroxidase level in the plant (Figure 4).
Resistance |
Clone |
Peroxidase (units min−1 g−1 fresh wt) |
Mean |
Non- Infected |
% IDOC |
Infected |
% IDOC |
Susceptible |
C9/2Kr-19 |
0.0163 t |
↓4.483 |
0.0283 q |
↓46.433 |
0.0255 B |
C9/2Kr-4 |
0.0207 r |
↑20.857 |
0.0353 mn |
↓33.207 |
C9/3Kr-38 |
- tu
|
↓10.331 |
0.0341 no |
↓35.448 |
C9/3Kr-45 |
0.0153 tu |
↓10.331 |
0.0313 p |
↓40.719 |
C9/3Kr-47 |
0.0187 s |
↑9.1618 |
0.0356 mn |
↓32.638 |
C9/3Kr-52 |
0.0143 u |
↓16.179 |
0.0352 mn |
↓33.428 |
C9/3Kr-56 |
0.0183 s |
↑7.2125 |
0.0328 op |
↓37.941 |
C9/13Kr-70 |
0.0187 s |
↑9.1618 |
0.0362 lm |
↓31.471 |
C9/1Kr-3 |
0.0193 u |
↑12.865 |
0.0371 op |
↓29.734 |
C9/3Kr-79 |
0.0143 u |
↓16.179 |
0.0326 op |
↓38.257 |
|
Mean |
0.0171 |
|
0.0338 |
|
Resistant |
C9 Cont |
0.0277 q |
|
0.0528 g |
|
0.0495 A |
C9/0.5Kr-11 |
0.0360 lmn |
↑29.963 |
0.0556 f |
↑5.2083 |
C9/0.5Kr-32 |
0.0360 lmn |
↑29.963 |
0.0567 f |
↑7.3864 |
C9/0.5Kr-42 |
0.0433 i |
↑56.438 |
0.0703 a |
↑33.207 |
C9/0.5Kr-46 |
0.0390 jk |
↑40.794 |
0.0568 f |
↑7.5442 |
C9/1Kr-3 |
0. 0377 kl |
↑35.980 |
0.0566 f |
↑7.2601 |
C9/2Kr-1 |
0.0457 h |
↑64.861 |
0.0596 e |
↑12.878 |
C9/3Kr-63 |
0.0393 jk |
↑41.997 |
0.0616 d |
↑16.635 |
C9/3Kr-69 |
0.0397 j |
↑43.201 |
0.0634 c |
↑20.138 |
C9/3Kr-8 |
0.0460 h |
↑66.065 |
0.0671 b |
↑27.020 |
|
Mean |
0.0390 |
|
0.0601 |
|
Mean |
0.0328 B |
|
0.0529 A |
|
|
LSD at (0.05) for: |
|
|
|
|
|
Resistance (R): |
0.00232 |
|
|
|
|
Infection (I): |
0.00613 |
|
|
|
|
Clones (C ): |
0.00232 |
|
|
|
|
RxIxC: |
0.0017 |
|
|
|
|
Table 4: Specific activity of Peroxidase (POX) in healthy and U. scitaminea infected sugarcane resistant and susceptible mutants.
*IDOC: % of Increase/Decrease over control, up arrows (↑) = increase down arrow, (↓) = decrease
*Different letters in means indicate a significant difference
POX: Peroxidase
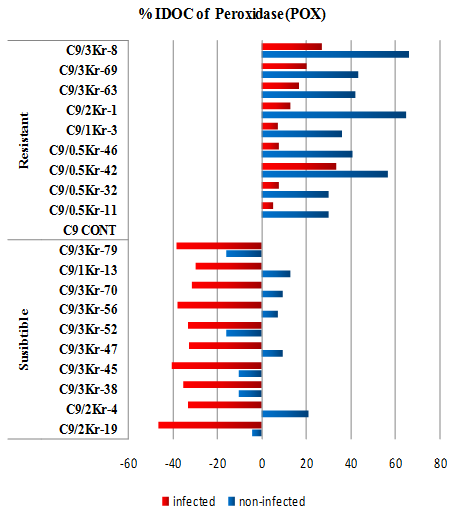
Figure 4: Percentage of Increase/Decrease over control (%IDOC) of Peroxidase (POX) in healthy and U. scitaminea infected sugarcane resistant and susceptible GT54-9 mutants.
In plants, the increased production of both the superoxide radical and H2O2 is a common feature of defence response to challenge by the microbial pathogen and elicitors [45]. It has been proposed that, the rapid increase in either intra or extra cellular H2O2 is involved in the induction of execution of the hypersensitive response [46]. Bestwick et al. [47] reported that, Cytochrome c peroxidase is a key enzyme during the synthesis of phytoalexin which has some inhibitory effect on disease.
Plant peroxidases can be directly involved in defence mechanisms acting as a catalyst for the polymerization of phenolic compounds to form lignin and suberin in the cell wall, which can act as mechanical barriers to block the spread of the pathogen in the plant [48]. The importance of peroxidases during plant resistance against pathogens has been demonstrated for the interaction between rice and Xanthomonas oryzae pv. oryzae [49] and between cotton and X. campestris pv. Malvacerum [50].
Another important difference was the enhancement in the resistant cultivar of peroxidase, an enzyme that uses free phenolics as substrates for the activation of the important mechanisms of resistance of sugar cane leaves to the fungal pathogen [51].
Turk [52] and Que et al. [53] reported that cytochrome c peroxidase is newly induced after infection, and the author believed that hydrogen peroxide redox type cytochrome c reaction (2 cytochrome c (Fe2+)+H2O2+2H+→2 cytochrome c (Fe3+)+2H2O) was catalyzed by the up-regulated expression of cytochrome c peroxidase, which improved the increasing synthesis of phytoalexin and inhibited the growth of S. scitamineum and thus reduced the harm of S. scitamineum.
Analysis of chitinase
Data presented in (Table 5) show that all the (SI) and (SNI) clones have a low level of chitinase compared to the infected and non infected control. The decrease percentage of chitinase over control IDOC in the susceptible clones ranged from 40.17% to 51.84%, while in the infected clones the decrease over control ranged from 52.89% to 67.67% (Figure 5).
Resistance |
Clone |
Chitinase (units min−1 g−1 fresh wt) |
Mean |
Non-Infected |
% IDOC |
Infected |
% IDOC |
|
Susceptible |
C9/2Kr-19 |
5.1288 o |
↓40.3043 |
13.7268 i |
↓59.3330 |
8.9131 B |
C9/2Kr-4 |
5.0156 o |
↓41.6216 |
15.7488 h |
↓53.3428 |
C9/3Kr-38 |
4.1374 p |
↓51.8439 |
12.6970 j |
↓62.3841 |
C9/3Kr-45 |
5.0831 o |
↓40.8367 |
15.9011 h |
↓52.8917 |
C9/3Kr-47 |
4.2670 p |
↓50.3352 |
13.1216 j |
↓61.1261 |
C9/3Kr-52 |
5.1407 o |
↓40.1660 |
11.0142 k |
↓67.3694 |
C9/3Kr-56 |
4.1642 p |
↓51.5317 |
13.7392 i |
↓59.2966 |
C9/3Kr-70 |
5.0872 o |
↓40.7887 |
10.9125 k |
↓67.6708 |
C9/1Kr-13 |
4.3564 o |
↓49.2947 |
11.3433 j |
↓66.3945 |
C9/3Kr-79 |
4.9858 o |
↓41.9693 |
12.6920 j |
↓62.3989 |
|
Mean |
4.7366 |
|
13.0897 |
|
Resistant |
C9 Cont |
8.5916 n |
|
33.7543e |
|
22.6195 A |
C9/0.5Kr-11 |
8.7170 n |
↑1.4599 |
34.1254 e |
↑1.0994 |
C9/0.5Kr-32 |
10.4055 l |
↑21.1131 |
31.0864 g |
↓7.9037 |
C9/0.5Kr-42 |
9.7082 m |
↑12.9969 |
37.0859 c |
↑9.8703 |
C9/0.5Kr-46 |
10.3956 l |
↑20.9971 |
36.1056 d |
↑6.9660 |
C9/1Kr-3 |
9.6148 m |
↑11.9091 |
30.8785 g |
↓8.5197 |
C9/2Kr-1 |
9.3880 m |
↑9.2698 |
32.9592 f |
↓2.3556 |
C9/3Kr-63 |
10.3986 l |
↑21.0317 |
40.0965 b |
↑18.789 |
C9/3Kr-69 |
8.6931 n |
↑1.1810 |
37.1152 c |
↑9.9569 |
C9/3Kr-8 |
10.3336 l |
↑20.2759 |
42.9358 a |
↑27.200 |
Mean |
9.6246 |
|
35.6143 |
|
|
Mean |
8.2437 B |
|
28.8700 A |
|
|
LSD at (0.05) for: |
|
|
|
|
|
Resistance (R): |
0.684 |
|
|
|
|
Infection (I): |
0.669 |
|
|
|
|
Clones (C ): |
0.684 |
|
|
|
|
RxIxC: |
0.504 |
|
|
|
|
Table 5: Specific activity of Chitinase in healthy and U. scitaminea infected sugarcane resistant and susceptible mutants.
*IDOC: % of Increase/Decrease over control, up arrows (↑) = increase down arrow, (↓) = decrease
*Different letters in means indicate a significant difference
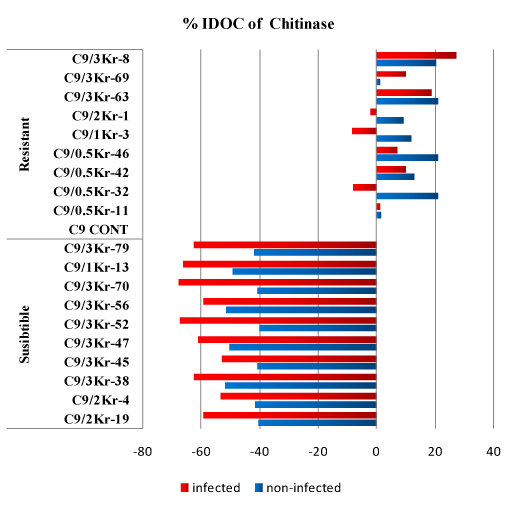
Figure 5: Percentage of Increase/Decrease over control (%IDOC) of Chitinase in healthy and U. scitaminea infected sugarcane resistant and susceptible GT54-9 mutants.
In resistant non infected clones the level of chitinase significantly increased in some of the tested clones while it was nonsignificant with the others compared to the non infected control (1.181% to 20.997%). In (RI) clones the level of chitinase increased over control in some clones and decreased in the others.
Analysis of β-1,3 glucanase
The results of the present study demonstrated that, the specific activity of β-1,3 glucanase showed similar pattern of decrease as for the activity of chitinase in the (SI) and (SNI) clones compared to the infected and non infected control (Table 6). In contrast to chitinase pattern of activity of the (RI) and (RNI) clones the specific activity of β-1,3 glucanase in all the tested colones decreased compared to the non infected control while in the infected clones some of the tested clones showed a reduction in the specific activity and the others showed increases (Figure 6).
Resistance |
Clone |
β 1,3 Glucanase (units min−1 g−1 fresh wt) |
Mean |
Non-Infected |
% IDOC |
Infected |
% IDOC |
Susceptible |
C9/2Kr-19 |
151.66 u |
↓21.00 |
189.57 n |
↓65.52 |
178.11 B |
C9/2Kr-4 |
145.68 v |
↓24.11 |
197.08 l |
↓64.16 |
C9/3Kr-38 |
151.72 u |
↓20.97 |
182.46 o |
↓66.82 |
C9/3Kr-45 |
154.66 t |
↓19.44 |
184.06 o |
↓66.53 |
C9/3Kr-47 |
165.71 r |
↓13.68 |
234.78 i |
↓57.31 |
C9/3Kr-52 |
154.66 tu |
↓19.44 |
194.75 lm |
↓64.58 |
C9/3Kr-56 |
148.73 v |
↓22.53 |
193.07 m |
↓64.89 |
C9/3Kr-70 |
146.70 v |
↓23.58 |
225.21 j |
↓59.04 |
C9/1Kr-13 |
153.64 t |
↓19.97 |
230.74 k |
↓58.04 |
C9/3Kr-79 |
155.67 t |
↓18.91 |
201.74 k |
↓63.31 |
|
Mean |
152.88 |
|
203.35 |
|
Resistant |
C9 Cont |
191.99 m |
|
549.97 f |
|
379.32 A |
C9/0.5Kr-11 |
173.10 q |
↓9.83 |
661.21 a |
↑20.22 |
C9/0.5Kr-32 |
166.03 r |
↓13.51 |
594.98 e |
↑8.18 |
C9/0.5Kr-42 |
158.08 s |
↓17.66 |
642.16 b |
↑16.76 |
C9/0.5Kr-46 |
174.99 q |
↓8.85 |
550.26 f |
↑0.05 |
C9/1Kr-3 |
173.07 pq |
↓9.85 |
631.14 c |
↑14.75 |
C9/2Kr-1 |
178.32 p |
↓7.11 |
483.09 h |
↓12.15 |
C9/3Kr-63 |
176.11 p |
↓8.26 |
526.53 g |
↓4.26 |
C9/3Kr-69 |
179.03 p |
↓6.74 |
591.98 e |
↑7.63 |
C9/3Kr-8 |
177.05 p |
↓7.78 |
607.33 d |
↑10.43 |
|
Mean |
174.78 |
|
583.87 |
|
Mean |
168.33 B |
|
478.55 A |
|
|
LSD at (0.05) for: |
|
|
|
|
|
Resistance (R): |
4.238 |
|
|
|
|
Infection (I): |
6.636 |
|
|
|
|
Clones (C ): |
4.238 |
|
|
|
|
RxIxC: |
3.122 |
|
|
|
|
Table 6: Specific activity of β-1,3 glucanase in healthy and U. scitaminea infected sugarcane resistant and susceptible mutants.
*IDOC: % of Increase/Decrease over control, up arrows (↑) = increase down arrow, (↓) = decrease
*Different letters in means indicate a significant difference

Figure 6: Percentage of Increase/Decrease over control (%IDOC) of β 1,3 glucanase in healthy and U. scitaminea infected sugarcane resistant and susceptible GT54-9 mutants.
Kuc [54] reveled that, the plant response phase against the infection includes the accumulation of different compounds such as phytoalexins (i.e. low molecular mass antimicrobial compounds that accumulate at sites of infection), systemic enzymes that degrade pathogens (e.g. chitinases, β-1,3-glucanases and proteases). Wu et al. [55] reported, two defense related genes encoding the anionic peroxidase and acidic chitinase were induced in transgenic Solanum tuberosum by the action of a broad range of fungal pathogens.
Mauch & Staehlin [56] reported that, Chitinase and glucanase are the most widely used approach of developing fungus resistant plants they found that, both enzymes showed over-expression in transgenic plants. They stated that, chitin and glucan comprise major components of the cell wall of most of the fungi. Over-expression of these hydrolytic enzymes in the plant cells is postulated to cause hyphal lysis, thereby inhibiting fungal growth. Broque et al. [57] constitutively expressed bean chitinase in tobacco and Brassica napus to enhance resistance towards Rhizoctonia solani. Among the PR proteins hydrolytic enzymes (chitinase and glucanase), Osmotins, Thionins and Defensins are specially important.
References
- Singh N, Somai BM, Pillay D (2004) Smut disease assessment by PCR and microscopy in inoculated tissue cultured sugarcane cultivars. Plant Science 167(5): 987-994.
- Martinez M, MedinaI I, Naranjo S, Rodriguez CW, de Armas R, et al. (2000) Changes of some chemical parameters, involved in sucrose recovery from sugarcane juices, related to the susceptibility or resistance of sugarcane plants to smut (Ustilago scitaminea). International Sugar Journal 102(1221): 445-448.
- Kosuge T (1969) The role of phenolics in host response to infection. Annual Review of Phytopathology 7: 195-222.
- Darvill AG, Albersheim P (1984) Phytoalexins and their elicitors, a defense against microbial infection in plants. Annual Review of Plant Physiology 35: 243-275.
- Broglie KR, Benhamou N, Chet I (1993) The role of cell wall degrading enzymes in fungal disease resistance. In: Chet I (Ed.), Biotechnology in Plant Disease Control. Wiley-Liss, New York, USA, pp. 139-156.
- Vance CP, Kirk TK, Sherwood RT (1980) Lignification as a mechanism of disease resistance. Annual Review of Phytopathology 18: 259-288.
- Espelie KE, Francheschi VR, Kolattukudy PE (1986) Immunocytochemical localization and time course of appearance of an anionic peroxidase associated with suberization inwound-healing potato tuber tissue. Plant Physiol 81(2): 487-492.
- Benhamou N, Mazau D, Grenier J, Tugay MTE (1991) Time-course study of the accumulation of hydroxyproline-rich glycoproteins in roots cells of susceptible and resistant plants infected by Fusarium oxysporum f.sp.radicis-lycopersici. Planta 184(2): 196-208.
- Van Loon LC, Gerritsen YAM (1989) Localization of pathogenesis-related proteins in infected and non-infected leaves of Samsun NN tobacco during the hypersensitive reaction to tobacco mosaic virus. Plant Sci 63(2): 131-140.
- Van Loon LE, Van Strien EA (1999) The families of pathogenesis related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55(2): 85-97.
- Datta K, Velazhahan R, Oliva N, Mew T, Muthukrishnan S, et al. (1999) Over-expression of thaumatin-like protein (PR-5) gene in transgenic rice plants enhances environmentally friendly resistance to Rhizoctonia solani causing sheath blight disease. Theor Appl Genet 98(6-7): 1138-1145.
- Legaz ME, de Armas R, Pinon D, Vicente C (1998) Relationships between phenolics-conjugated polyamines and sensitivity of sugarcane to smut (Ustilago scitaminea). Journal of Experimental Botany 49(327): 1723-1728.
- Pinon D, de Armas R, Vicente C, Legaz ME (1999) Role of polyamines in the infection of sugarcane buds by Ustilago scitaminea spores. Plant Physiol Biochem 37(1): 57-64.
- Fontaniella B, Marquez A, Rodriguez CW, Pinon D, Solas MT, et al. (2002) A role for sugarcane glycoproteins in the resistance of sugarcane to Ustilago scitaminea. Plant Physiology and Biochemistry 40(10): 881-889.
- Millanes AM, Fontaniella B, Legaz ME, Vicente C (2005) Glycoproteins from sugarcane plants regulate cell polarity of Ustilago scitaminea teliospores. J Plant Physiol 162(3): 253-265.
- Legaz ME, Armas R de, Millanes AM, Rodriguez CW, Vicente C (2005) Heterofructans and heterofructan containing glycoproteins from sugarcane: structure and function. Research and Development Biochemistry 6: 31-51.
- Gillaspie AG, Mock RG, Dean JL (1983) Differentiation of Ustilago scitamenea isolates in greenhouse tests. Plant disease 67(4): 373-375.
- Trione EJ (1990) Growth and sporulation of Ustilago scitaminea,in vivoand in vitro. Mycological Research 94(4): 489-493.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Malik CP, Singh MB (1990) Extraction and estimation of amino acids and kito acids. In: Plant Enzymology and histo-Enzymology. New Delhi-Lud Hana, India.
- Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenol oxidase activities during rice leaf senescence. Plant Physiol 57(2): 315-319.
- Zucker M (1968) Sequential induction of phenylalanine ammonia-lyase and a lyase inactivating system in potato tuber disks. Plant Physiol 43(3): 365-374.
- Saunders JA, McClure JW (1974) The suitability of a quantitative spectrophotometric assay for phenylalanine ammonia‑lyase activity in barley, buckwheat and pea seedlings. Plant Physiol 54(3): 412‑413.
- Van Asperen K (1962) A study of housefly esterases by means of a sensitive colorimetric method. J Insect Physiol 8(4): 401-416.
- Reissig JL, Stringer JL, Leloir LF (1955) A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem 217(2): 959-966.
- Singh RP, Singh US (1994) Molecular methods in plant pathology. Lewis Press Inc, CRC Boca Raton, London, Tokyo, pp. 58.
- Naguib MI (1964) Effect of seivn on the carbohydrate and nitrogen metabolism during the germination of cotton seeds. Ind J Agric Sci 35: 179-185.
- Naguib MI (1965) Effect of maleic hydrazine on the nitrogen metabolism during the germination of cotton seeds. Ind J Exp Biol 2: 149-152.
- Snedecore GW, Cochran WG (1982) Statistical methods. (7th edn), Iowa state University, Pres Ames, USA.
- SPSS Inc (1998) SPSS Base 8.0 for Windows User's Guide. SPSS Inc., Chicago, Illinois, USA.
- Waller JM (1970) Sugarcane smut (Ustilago scitaminea) in Kenya: Infection and resistance. Transactions of the British Mycological Society 54(3): 405-414.
- Alexander KC, Ramakrishnan K (1980) Infection of the bud, establishment in the host and production of whips in sugarcane smut (Ustilago scitaminea) of sugarcane. Proceedings of the International Society of Sugarcane Technology 17: 1452-1455.
- Vaughan KC, Duke SO (1984) Function of polyphenol oxidase in higher plants. Physiologia Plantarum 60(1): 106-112.
- Aniszewski T, Lieberei R, Gulewicz K (2008) Research on catecholases, laccases and cresolases in plants. Recent progress and future needs. ACTA Biologica Cracoviensia Series Botanica 50(1): 7-18.
- Agrias GN (1997) Plant Pathology. Indian reprints. Replika Press Pvt. Ltd., India, 4(7): pp.106-108.
- Sundar AR, Viswanathan R, Malathi P, Padmanaban P (2006) Mechanism of resistance induced by plant activators against Colletotrichum falcatum in sugarcane. Archives of Phytopathology and Plant Protection 39(4): 259-272.
- De Armas R, Santiago R, Legaz ME, Vicente C (2007) Levels of phenolic compounds and enzyme activity can be used to screen for resistance of sugarcane to smut (Ustilago scitaminea). Australasian Plant Pathology 36(1): 32-38.
- Singh J, Singh M, Chandra P, Rao GP, Singh HN (1993) Biochemical studies on resistance to red rot in sugarcane. Sugarcane 6: 16-19.
- Chylinska KM, Knypl JS (1975) Decreased phenylalanine ammonia lyase and ribonuclease activity in side roots of carrot infested with northern root-knot nematode. Nematologica 21(2): 129-133.
- Ouyang YX, Kui LZ, Yun LC (2000) Physiological and biochemical characterization of in vitro screened rice somaclonal variants resistant to Xanthomonas oryzae. Acta Phytopathologica Sinica 30(1): 42-47.
- Lian HG, Wu CG, Jie C, Jing F, Shu C, et al. (2005) The function of phenolic metabolism in resistant mechanism of gray leaf spot of corn. Acta Phytopathologica Sinica 33(4): 342-346.
- Kim YS, Lee HH, Ko MK, Song CE, Bae CY, et al. (2001) Inhibition of fungal appressorium formation by pepper (Capsicum annum) esterase. Mol Plant Microbe Interact 14(1): 80-85.
- Zhang LH, Birch RG (1997) The gene for albicidin detoxification from Pantoea dispersa encodes an esterase which attenuates pathogenicity of Xanthomonas albilineas in sugarcane. Proc Natl Acad Sci USA 94(18): 9984-9989.
- Koretsky LS (2001) The influence of Fusarium oxysporum infection and low temperatures on the activity of soybean esterase and PR proteins. Buvisindi 14: 67-73.
- Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48: 251-275.
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidant burst orchestrates the plant hypersensitive disease resistance response. Cell 79(4): 583-593.
- Bestwick CS, Brown IR, Bennett MHR, Mansfield JW (1997) Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9(2): 209-221.
- Lebeda A, Luhova L, Sedlarova D, Jancova D (2001) The role of enzymes in plant-fungal pathogens interactions. Journal of Plant Diseases Protection 108: 89-111.
- Chittor JM, Leac JE, White FF (1997) Differential induction of a peroxidase gene family during infection of rice by Xanthomonas oryzae pv. oryzae. Mol Plant Microbe Interact 10(7): 861-871.
- Dai GH, Nicole M, Andary C, Martinez C, Bresson E, et al. (1996) Flavonoids accumulate in cell walls, middle lamellas and callose-rich papillae during an incompatible interaction between Xanthomonas campestris pv. malvacearum and cotton. Physiol Mol Plant Pathol 49(5): 285-306.
- Santiago RL, de Armas RM, Legaz E, Vicente C (2008) Separation from Ustilago scitaminea of different elicitors which modify the pattern of phenolic accumulation in sugarcane leaves J Plant Pathol 90(1): 87-96.
- Turk FM (2002) Phytoalexins: Defense or just a response to stress? J Cell Molecul Biol 1: 1-6.
- Que YX, Yang ZX, Xu LP, Chen RK (2009) Isolation and identification of differentially expressed genes in sugarcane infected by Ustilago scitaminea. Acta Agronomica Sinica 35(3): 452-458.
- Kuc J (1990) Compounds from plants that regulate or participate in disease resistance. Ciba Found Symp 154: 213-224.
- Wu G, Shortt BJ, Lawrence EB, Leon J, Fitzsimmons KC, et al. (1997) Activation of host defense mechanisms by elevated production of H2O2 in transgenic plants. Plant Physiol 115(2): 427-435.
- Mauch F, Staehelin LA (1989) Functional implications of the subcellular localization of ethylene-induced chitinase and ß-1,3-glucanase in bean leaves. Plant Cell 1(4): 447- 457.
- Broque K, Chet I, Holliday M, Cressman R, Biddle P, et al. (1991) Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science 254(5035): 1194-1197.

©2014 Esh, et al. This is an open access article distributed under the terms of the,
which
permits unrestricted use, distribution, and build upon your work non-commercially.








