Advances in
eISSN: 2373-6402


Review Article Volume 4 Issue 6
Department of Plant Physiology, Institute of Agricultural Sciences, Banaras Hindu University, India
Correspondence: Deepmala Katiyar, Department of Plant physiology, Institute of Agricultural Sciences Banaras Hindu University, Uttar Pradesh, India, Tel +054-267-029-390 +91945- 226-7383
Received: September 25, 2016 | Published: October 27, 2016
Citation: Katiyar D, Hemantaranjan A, Singh B. Plant growth promoting Rhizobacteria-an efficient tool for agriculture promotion. Adv Plants Agric Res. 2016;4(6):426-434. DOI: 10.15406/apar.2016.04.00163
Current soil management strategies are mainly dependent on inorganic chemical-based fertilizers, which caused a serious threat to human health and environment. Plant growth-promoting rhizobacteria (PGPR) are naturally occurring soil bacteria that aggressively colonize plant roots and benefit plants by providing growth promotion. Inoculation of crop plants with certain strains of PGPR at an early stage of development improves biomass production through direct effects on root and shoots growth. The major groups of PGPR can be found along with the phyla actinobacteria, bacteroidetes, firmicutes, and proteobacteria. Inoculation of agricultural crops with PGPR may result in multiple effects on early-season plant growth, as seen in the enhancement of seedling germination, plant health, vigor, height, shoot weight, nutrient content of shoot tissues, early bloom, chlorophyll content, and increased nodulation in legumes. PGPRs are reported to influence the growth, yield, and nutrient uptake by an array of mechanisms. They help in increasing nitrogen fixation in legumes, help in promoting free-living nitrogen-fixing bacteria, increase supply of other nutrients, such as phosphorus, iron and produce plant hormones that enhance other beneficial bacteria or fungi. Now a day’s an increasing number of PGPR being commercialized for various crops. Subsequently, there has been much research interest in PGPRs. Several reviews have discussed specific aspects of growth promotion by PGPRs. Therefore, PGPRs can help to generate wealth cooperatively in local communities, reducing the need for more expensive manufactured products, such as nitrogenous fertilizers and use of PGPR in world has the potential to provide valuable insight.
Keywords: Plant growth promoting rhizobacteria (PGPR), growth, agriculture, boifertilizers
Conventional agriculture plays a significant role in meeting the food demands of a growing human population; this has also led to an increasing dependence on chemical fertilizers.1 As agricultural production strengthened over the past few decades, farmers became more and more dependent on chemical fertilizers as a relatively reliable method of crop protection helping with economic stability of their manoeuvre. Chemical fertilizers are industrially manipulated substances composed of known quantities of nitrogen, phosphorus and potassium, and their exploitation causes air and ground water pollution by eutrophication of water bodies.2 Nevertheless, increasing use of chemical inputs causes several negative effects, i.e., development of pathogen resistance to the applied agents and their non target environmental impacts.3,4 An ample assortment of agriculturally important microorganisms have been taken use of crop health and production management, which comprise nitrogen fixers like Rhizobium, Bradyrhizobium, Sinorhizobium, Azotobacter, Azospirillum, phosphate solubilisers like Bacillus, Pseudomonas, Aspergillus, Enterobacter and Arbuscular mycorrhizae in agriculture. They are well known to increase plant growth, induce host plant resistance and crop yield.5 The rhizosphere region has been distinct as the volume of soil directly influenced by the presence of living plant roots or soil compartment influenced by the root.6 Rhizosphere supports large and active microbial population capable of exerting beneficial, neutral and detrimental effects on the plants. Various free-living soil bacteria that are capable of applying beneficial effects on plants in culture or in a protected environment via direct or indirect mechanisms.7,8 The focus of this review is potential of PGPR which act as biofertilizers, either directly by helping to provide nutrient to the host plant, or indirectly by positively influencing root growth and morphology or by aiding other beneficial symbiotic relationships.
Now a day an agricultural production can be increased efficiency by fertilization and it is only way for recovery of production. Non-organic synthetic fertilizers mainly contain phosphate, nitrate, ammonium and potassium salts. Fertilizer used to add nutrients to the soil to promote soil fertility and increase plant growth. They reduce the food value of plants. The nutrient reservoirs in the soil shrink when crops are removed from the field at harvest. This nutrient export creates a phosphorus deficit, necessitating regular phosphorus addition to replace the harvested phosphorus. This leads to the need of frequent application of chemical phosphate fertilizers, but its use on a regular basis has become a costly affair and also environmentally undesirable.9 The excessive use of chemical fertilizers in plants not only affects the quality of food but also environment. Fertilizer industry is considered to be source of natural radionuclides and heavy metals as a potential source. It contains a large majority of the heavy metals like Cd, Pb, Hg and as10,11 and some results in the accumulation of inorganic pollutants.12 Plants absorb the fertilizers through the soil; they can enter the food chain. Thus, fertilization leads to water, soil and air pollutions. In recent years, fertilizer consumption increased continuously throughout the world, causes severe environmental problems as well as many diseases in human like Stomach cancer, goiter, and several vector borne diseases. In infants it is the reason of blue baby syndrome. It also leads to groundwater contamination.13 There are also a number of fastidious diseases for which chemical solutions are few and ineffective.14 Biological control is thus being considered as an alternative or a supplemental way of reducing the use of chemicals in agriculture.15
The narrow zone of soil directly surrounding the root system is referred to as rhizosphere,16 while the term ‘rhizobacteria’ implies a group of rhizosphere bacteria competent in colonizing the root environment.17 About 2–5% of the rhizosphere bacteria are PGPR.18 The term PGPR was coined by Joe Kloepper in late 1970s and was defined by Kloepper et al.,19 as ‘‘the soil bacteria that colonize the roots of plants by following inoculation on to seed and that enhance plant growth’’. The rhizosphere, volume of soil surrounding roots and influenced chemically, physically and biologically by the plant root, is a highly favourable habitat for the proliferation of microorganisms and exerts a potential impact on plant health and soil fertility.20 Root exudates rich in amino acids, monosaccharides and organic acids, serve as the primary source of nutrients, and support the dynamic growth and activities of various microorganisms within the vicinity of the roots.21 On the basis of their location in rhizosphere PGPR can be classified as extracellular PGPR found in the rhizosphere, on the rhizoplane or in the spaces between the cells of the root cortex and intracellular PGPR which exist inside the root cells, generally in specialized nodular structures.22 PGPR represent a wide variety of soil bacteria which grown in association with a host plant, result in stimulation of growth of their host. PGPR have the potential to contribute in the development of sustainable agricultural systems. In general, PGPR function in three different ways:23,24 synthesizing particular compounds for the plants25,26 facilitating the uptake of certain nutrients from the soil27 and preventing the plants diseases,28,29 (Figure 1).
Wide ranges of bacterial groups being considered as plant growth promoting rhizobacteria include Acinetobacter, Agrobacterium, Arthobacter, Azotobacter, Azospirillum, Burkholderia, Bradyrhizobium, Rhizobium, Serratia, Thiobacillus, Pseudomonads, and Bacilli in various plants,30,31 (Table 1).
Plant growth promoting rhizobacteria (PGPR) |
Crops |
Plant growth promoting traits |
Literature cited in |
Azospirillum sp. |
Rice |
Nitrogen fixation |
|
Paenibacillus polymyxa |
Wheat |
Cytokinin |
|
Pseudomonas rathonis |
Wheat, Maize |
Auxin production |
|
Comamonas acidovorans |
Lettuce |
IAA production |
|
Azoarcus sp. |
Kallar grass |
Nitrogen fixation |
|
Kluyvera ascorbata |
Canola, tomato |
Siderophores, |
|
Azotobacter sp. |
Sesbenia, |
IAA production |
|
Pseudomonas fluorescens |
Soybean |
Cytokinin |
|
Azoarcus sp. |
Rice |
Nitrogen fixation |
|
Enterobacter cloacae |
Rice |
IAA production |
|
Pseudomonas sp. |
Mungbean |
IAA production |
|
Alcaligenes sp. |
Rape |
ACC deaminase |
|
Azoarcus sp. |
Sorghum |
Nitrogen fixation |
|
Rhizobacterial isolates |
Wheat, rice |
Auxin production |
|
Enterobacter sp. |
Sugarcane |
IAA production |
|
Pseudomonas sp. |
Wheat |
IAA production |
|
Azotobacter sp. |
Maize |
Nitrogen fixation |
|
Pseudomonas fluorescens |
Pine |
Cytokinin |
|
Rhizobium leguminosarum |
Rice |
IAA production |
|
Pseudomonas sp. PS1 |
Greengram |
Phosphate solubilization, Nitrogen fixation |
|
Bacillus cereus RC 18, |
Wheat |
IAA production |
|
Streptomyces, anthocysnicus, Pseudomonas aeruginosa, Pseudomonas pieketti |
Rice |
IAA production |
|
Rhizobium leguminosarum |
Rape & lettuce |
Cytokinin |
|
Bacillus licheniformis C08 |
spinach |
IAA production |
|
Rhizobium leguminosarum |
Radish |
IAA production |
|
Azotobacter sp. |
Wheat |
Nitrogen fixation |
|
Azotobacter sp. |
Maize |
IAA production |
|
Mesorhizobium loti MP6, Pseudomonas fluorescens |
Brassica |
Siderophore, |
|
Pseudomonas tolaasii |
Brassica |
Siderophores, |
|
Bacillus polymyxa |
Wheat |
Nitrogen fixation |
|
Bacillus pumilus |
Rape |
ACC deaminase |
|
Pseudomonas fluorescens |
Groundnut |
Siderophores, |
|
Bacillus sp. |
Alder |
Gibberellin |
|
Bacillus sp. |
Rice |
IAA production |
|
Burkholderia sp. |
Rice |
Nitrogen fixation |
|
Azospirillum lipoferum |
Wheat |
IAA production |
|
Pseudomonas putida, Azospirilium, Azotobacter |
Artichoke |
Phosphate solublization |
|
Gluconacetobacter diazotrophicus |
Sorghum |
Nitrogen fixation |
|
Azospirillum brasilense |
Wheat |
IAA production |
|
Enterobacter cloacae |
Rape |
ACC deaminase |
|
Streptomyces acidiscabies |
Cowpea |
Hydroxamate |
|
Gluconacetobacter diazotrophicus |
Sugarcane |
Nitrogen fixation |
|
Pseudomonas sp. |
Rape |
ACC deaminase |
|
Aeromonas veronii |
Rice |
IAA production |
|
Bradyrhizobium sp. |
Radish |
IAA production |
|
Pseudomonas cepacia |
Soybean |
ACC deaminase |
|
Herbaspirillum sp. |
Rice |
Nitrogen fixation |
|
Variovorax paradoxus |
Rape |
ACC deaminase |
|
Herbaspirillum sp. |
Sorghum |
Nitrogen fixation |
|
Agrobacterium sp. |
Lettuce |
IAA production |
|
Pseudomonas putida |
Mung bean |
ACC deaminase |
|
Herbaspirillum sp. |
Sugarcane |
Nitrogen fixation |
|
Alcaligenes piechaudii |
Lettuce |
IAA production |
|
Burkholderia verschuerenni Burkholderia sp. |
Sugarcane |
IAA production |
Table 1 Plant growth promoting rhizobacteria (PGPR) for which evidence exists that their stimulation of plant growth promoting traits in numerous crops
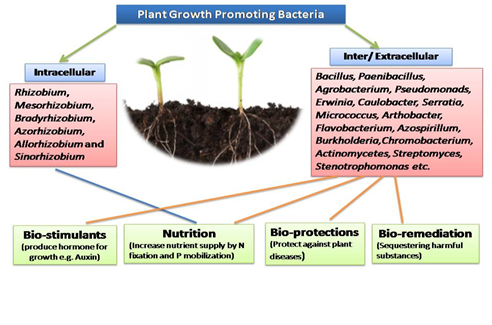
The potential use of biofertilizers is now being seriously considered as a means to reduce the quantity of fertilizers required for crop production. This would help to minimize pollution and soil infertility, and above all reduce grower’s costs. PGPR have been reported to be present in high populations, in the rhizosphere and as endophytes of many crops. They include species of Enterobacter, Bacillus, Klebsiella, Herbaspirillum, Burkholderia, Azospirillum, and Gluconacetobacter.32 The most common bacteria isolated from sugarcane tissues have been Gluconacetobacter diazotrophicus, Herbaspirillum rubrisubalbicans, and H. seropedicae,33 whereas Enterobacter cloacae, Erwinia herbicolla, K. pneumoniae, K. oxytoca, Azotobacter vinelandii, Paenibacillus polymyxa, and Azospirillum were found less often.33
The growth promotion channel by these bacteria that enhances the plant growth was not fully known while in few ways it is understood.34 The well known mechanism for the growth promotion is through producing various plant growth hormones that include Gibberellin and Indole-3-acetic acid (IAA) Arshad23,35 solubilisation of insoluble phosphate36 fixation of atmospheric nitrogen37,38 and hore synthesis39 hydrogen cyanide production40 and various antagonistic activity against the plant pathogens.41 Therefore it is necessary to develop a rhizobacterial population that encompasses significant plant growth role for the improvement of agricultural practices and yield, thereby reducing the application of chemical biofertilizer and chemical pesticides, the present study was focused in the path to isolate an efficient PGPR strain from the rhizosphere of sugarcane plant and to assess the plant growth promoting activities.
Taxonomy is defined as the science dedicated to the study of relationships among organisms and has to do with their classification, nomenclature, and identification42 The accurate comparison of organisms depends on a reliable taxonomic system. Even though many new characterization methods (including gene content, sequences of conserved macromolecules, gene order, dinucleotide relative abundance values and codon usage) have been developed over the last 30years and used to study phylogenetic relationships between bacterial taxa.43
Biofertilizers, more commonly known as microbial inoculants, are artificially multiplied cultures of certain soil organisms that can improve soil fertility and crop productivity. Although the beneficial effects of legumes in improving soil fertility was known since ancient times and their role in biological nitrogen fixation was discovered more than a century ago, commercial exploitation of such biological processes is of recent interest and practice. The commercial history of biofertilizers began with the launch of ‘Nitragin’ by Nobbe and Hiltner, of Tharand, Germany, have invented certain new and useful improvements relating to the Inoculation of soil for the cultivation of leguminous plants and a laboratory culture of rhizobia in 1895, followed by the discovery of Azotobacter and then the blue green algae. Azospirillum and Vesicular- Arbuscular Micorrhizaeare fairly recent discoveries. In India the first study on legume rhizobium symbiosis was conducted by N.V. Joshi and the first commercial production started as early as 1956. However the Ministry of Agriculture under the ninth plan initiated the real effort to popularize and promote the input with the setting up of the National Project on Development and Use of Biofertilizers (NPDB). Commonly explored biofertilizers in India are mentioned below along with some salient features. Recently PGPR have attracted the attention of agriculturists as soil inoculums to improve plant growth and yield.44 Significant increases in growth and yield of agronomically important crops in response to inoculation with PGPR have been repeatedly reported.45–55 Studies have also shown that the growth-promoting ability of some bacteria may be highly specific to certain plant species, cultivar and genotype.56–58 Plant growth-promoting rhizobacteria are the rhizospheric bacteria that can enhance plant growth by a wide variety of activities like.
Phosphorus, both native in soil and applied in inorganic fertilizers becomes mostly unavailable to crops because of its low levels of mobility and solubility and its tendency to become fixed in soil. The phosphate sulubilizing (PSB) bacteria are life forms that can help in improving phosphate uptake of plants in different ways. The PSB also has the potential to make utilization of India’s abundant deposits of rock phosphates possible, much of which is not enriched. PSB are group of beneficial bacteria capable of hydrolyzing organic and inorganic phosphorus from insoluble compounds.59 Phosphate solubilization ability of the micro-organisms is considered to be one of the most important traits associated with plant phosphate nutrition.60 It is generally accepted that mechanisms of the mineral phosphate solubilization by the PSB strains is associated with the release of low molecular weight organic acids, through which their hydroxyl and carboxyl groups chelate the cations bound to phosphate there by converting it into soluble forms.61 The PGPR occur in soil, usually their number are not high enough to compete with other microorganisms commonly established in the rhizosphere.
Thus the amount of P liberated by them is generally not sufficient for a substantial increase of in situ plant growth. Therefore inoculation of plants by a target microorganism at a much higher concentration than the normal found in soil is necessary to take advantage of the property of phosphate solubilization for plant yield enhancement.62 Inoculation of PGPR in the soil is a promising technique because it can increase phosphorous availability63 and improves the physio-chemical, biochemical and biological properties of soil.64 So that use of PGPR in agriculture can not only compensate for higher cost of manufacturing fertilizers in industries but also mobilizes the fertilizers added to soil. In addition some PSB produce phosphatase like phytase that hydrolyse organic forms of phosphate compound efficiently.
About 78% of the earth atmosphere is made up of free nitrogen (N2) produced by biological and chemical processes within the biosphere and not combined with other elements. All plants need nitrogen for their growth. However plants cannot get the nitrogen they need from atmospheric supply. They can use only nitrogen that is available in compound form. Nitrogen occurs in the atmosphere as N2, a form that is not useable by plants. Nitrogen fixation is the first major mechanism for the enhancement of plant growth by Azospirillum.65 Azospirillum species are aerobic heterotrophs that fix N2 under microaerobic conditions66 and grow extensively in the rhizosphere of gramineous plants.67,68 The Azospirillum–plant association leads to enhanced development and yield of different host plants.67 This increase in yield is attributed mainly to an improvement in root development by an increase in water and mineral uptake, and to a lesser extent biological N2-fixation.68,69
Iron is an essential nutrient for almost all forms of life. All microorganisms known so far, with the exception of certain lactobacilli, essentially require iron.70 In the aerobic environment, iron occurs principally as Fe3+ and is likely to form insoluble hydroxides and oxyhydroxides, thus making it generally inaccessible to both plants and microorganisms. Despite being one of the most abundant elements in the earth’s crust, the bioavailability of iron in many environments such as the soil is limited by the very low solubility of the Fe3+ ion. It accumulates in commercial mineral phases such as iron oxides and hydroxides71 therefore cannot be readily utilized by the organisms. Microbes release siderophores to scavenge iron from these mineral phases by formation of soluble Fe3+ complexes that can be taken up by the active transport mechanisms. Bacteria acquire iron by the secretion of low-molecular mass iron chelators referred to as siderophores which have high association constants for complexing iron. Most of siderophores are small, water soluble, high affinity iron chelating compounds amongst the strongest soluble Fe3+ binding agents known.72 Thus, siderophores act as solubilizing agents for iron from minerals or organic compounds under conditions of iron limitation.73 A great deal of evidence exists that a number of plant species can absorb bacterial Fe3+ siderophore complexes, and this process is vital in absorption of iron by plants.74
PGPRs produce plant hormones both in liquid cultures and natural condition. The major hormones produced are Indole acetic acid (IAA).75 It is reported that 80% of microorganisms isolated from the rhizosphere of various crops possess the ability to synthesize and release auxins as secondary metabolites.76 IAA plays a very important role in rhizobacteria-plant interactions.77 The IAA synthesized by PGPRs influenced the root hair development, respiration rate, metabolism and root proliferation which in turn resulted in better mineral uptake of the inoculated plants.78 IAA formation via indole-3-pyruvic acid and indole-3-acetic aldehyde is found in a majority of bacteria like, Erwinia herbicola; saprophytic species of the genera Agrobacterium and Pseudomonas; certain representatives of Bradyrhizobium, Rhizobium, Azospirillum, Klebsiella, and Enterobacter. Most Rhizobium species have been shown to produce IAA.79
Biological N2 fixation represents the major source of N input in agricultural soils including those in arid regions. The major N2-fixing systems are the symbiotic systems, which can play a significant role in improving the fertility and productivity of low-N soils. The Rhizobium-legume symbioses have received most attention and have been examined extensively.80 These Rhizobia (species of Rhizobium, Mesorhizobium, Bradyrhizobium, Azorhizobium, Allorhizobium and Sinorhizobium) inoculants are known for their ability to fix atmospheric nitrogen in symbiotic association with legume by responding chemotactically to flavonoid molecules released as signals by the legume host. These plant compounds induce the expression of nodulation (nod) genes in rhizobia, which in turn produce lipo-chitooligiosaccharide signals that trigger mitotic cell division in roots, leading to nodule formation.81–83 The legume-Rhizobium symbiosis is a typical example of mutualism, but its evolutionary persistence is actually somewhat surprising. Because several unrelated strains infect each individual plant, any one strain could redirect resources from N2 fixation to its own reproduction without killing the host plant upon which they all depend.84–90 It turns out that legume plants guide the evolution of rhizobia towards greater mutualism by reducing the oxygen supply to nodules that fix less N2 thereby reducing the frequency of cheaters in the next generation. Symbiotic N2-fixation has been studied widely and exploited as a means of increasing crop yields,91–94 but rhyzobium are however limited by their specificity and only certain legumes are benefited from this symbiosis.94–100
This review has shown that there is huge potential for the use of PGPRs as biofertilizing agents for a wide variety of crop plants.97 For this reason, there is an urgent need for research to clear definition of what bacterial traits are useful and necessary for different environmental conditions and plants.101–105 They must be exploited to develop eco-friendly and safe replacement for chemical based fertilizers. Therefore, efficient PGPR strains can either be selected or improved.106,107 The success of the science related to biofertilizers depends on inventions of innovative strategies related to the functions of PGPRs and their proper application to the field of agriculture.108–110 The major challenge in this area of research lies in the fact that along with the identification of various strains of PGPRs and its properties it is essential to dissect the actual mechanism of functioning, synergistic effects of PGPRs for their efficacy toward exploitation in sustainable agriculture.111–115 However, the triumph in developing PGPRs mediated tools is greatly dependent on the development of efficient and sensitive molecular genetics techniques like microarrays and effective culturing methodologies to provide a better insight of the structural and functional diversity of the rhizosphere.116–121 Design of economically feasible large scale production methodologies and inoculation technologies are thus other critical requirements. So, deep rooted research in this area is highly needed. PGPRs are the potential tools for sustainable agriculture and trend for the future.
The Authors wish to thank University Grant Commission, New Delhi for financial support in form of Post Doctoral Fellowship (F.15-1/2012-13/PDFWM-2012-13-OB-UTT-17432). We are thankful to Department of Plant physiology, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi for providing necessities of this work.
The author declares no conflict of interest.

©2016 Katiyar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.