Advances in
eISSN: 2373-6402


Research Article Volume 7 Issue 5
1Department of Biological Sciences, Swat College of Science and Technology Swat, Pakistan
2Department of Biotechnology, University of Malakand, Chakdara, Pakistan
3Department of Biotechnology, Sarhad University Peshawar, Pakistan
Correspondence: Ayaz Ahmad, Department of Biological Sciences, Swat College of Science and Technology Swat, Khyber-Pakhtunkhwa, Pakistan
Received: May 04, 2017 | Published: October 10, 2017
Citation: Ahmad A, Hadi F, Ali N. Phytoextraction and translocation of cadmium in saline soil by hemerocallis fulva and dodonaea viscosa plants. Adv Plants Agric Res. 2017;7(5):397-405. DOI: 10.15406/apar.2017.07.00273
Purpose: Cadmium contaminated Saline soil has always been a problem for sustainable agriculture and environment. Cadmium (Cd) is a noxious heavy metal and its co-occurrence with high salt (NaCl) concentrations in soil decreases quality of food and quantity of crops. For this purposes to search the salinity problems in Cd contaminated soil, its uptake and accumulation and its effect on plant growth and biomass were studied in two terrestrial plants.
Materials and methods: Different concentration of salt, NaCl (1000, 3000 and 6000ppm) in combination with Cd metal (50, 100 and 150ppm) were added into pots soil, the two plants (Hemerocallis fulva and Dodonaea viscosa) were grown in it. For control (C) is used having no cadmium and salt while the remaining three with diverse concentrations of Cd (C1=50ppm, C2=100ppm and C3=150ppm).
Results and Discussion: Plant biomass and growth were highly reduced under variable concentrations of Cd and salt in soil. Combination of 6000ppm NaCl and 150ppm Cd in soil demonstrated highest significant Cd accumulation in the plants. Dodonaea viscosa showed high Cd-bio concentration value (more than one) as compared to Hemerocallis fulva having less than one. It was noted that Dodonaea viscosa plant accumulates maximum concentration of Cd in sodium salt than Hemerocallis fulva plant.
Conclusion: Dodoneae plant potentially hyper accumulator and showed enough tolerance to high concentration of salt during Phytoextraction of Cd. It is strongly recommended that such plants should be planted in metal contaminated saline soil and also for the conservation of barren soil.
Keywords: terrestrial plants, salinity, soil, phytoextraction
Availability of clean and fresh water is a global issue, environmental threat, life span indicator, remedy to cell metabolism and physiological activities are totally depend upon the fresh water. Globally high salt concentration in the soil is a major issue for arid, semi-arid and oceanic region in the world.1,2 It generates responses in plants, changing in oxidation reduction processes in the cell, plant yield and growth as well as biomass3,4 Such environmental stress conditions influence the root relation with surrounding and ascent poisonous materials within the plants .World widely 955M ha, soil is primary affected by salt while increasing rate of salinity secondary affect 77M ha, in which 58% is through irrigation. In Asia there is approximately 21.5million hectare soil is salt affected by major ions like Na+, K+, Ca++, Mg++ and Cl-. Hyper salinization severely impact on crops to reduce growth, yields and productivity because it is a vital environmental stress.5 Salt concentrations also influence seedling, cellular plant metabolism due to osmotic stress, oxidative stress and particular effects of ions.6
From the collected data revealed that some of the plants are very tolerance to salinity specially Barley, chick pea.7 In Pakistan, majority places in plane areas containing maximum amount of salt in soil than needed, such soil is physiologically draught and barren while worthless for cultivation of crops, vegetables and other plants and also produced environmental issue for the people and countries. Other than some soils also having toxic metals which further make soil noxious for cultivation and crops purposes. Salt stress is an environmental issue for control process of limited resource of plant growth, production, distribution and abundance of a population. In recent survey report estimated that majority of irrigation water is either alkaline or saline about one third of irrigation section8 Cadmium circulation rate is high and easily be absorbed and translocated by plants because its low soil holding capacity.9 Cadmium is so toxic when enters into living through food chain can generate chronic health problems.10
The regulatory limit of cadmium (Cd) in agricultural soil is 100mg/kg soil,11 but this threshold is continuously exceeding because of several human activities. Plants exposed to high levels of Cd causes reduction in photosynthesis, water uptake, and nutrient uptake. Hypertonic salt solution in soil decrease germination of seeds, seedling, flowering and fruit formation, quantity and quality of crops.12 Maximum amount or above the normal amount of salt develops osmotic shock disturbs ionic concentration in plant cells,13 which ultimately denature physiologically activities of plants on cellular level as well as on the whole plants by osmotic and ionic stress. Dissolved salts make isotonic environment for crops, but some plants are tolerate to salt, metals and enable to absorb maximum amount of metals along with salts, simply to absorb maximum amount of metals along with salts, from naturally saline substrates like bio solids (organic nutrients prepared from the treatment of manure ,thick, soft and wet mud as well as industrial or refinery sludge. According to the report of,14 Plants adopt their selves with water stress and salt stress condition through osmosis by intake or lose of substances to maintain their status due to change the internal photosynthetic enzymatic quantity and balance because of external shade and light stress (helio and hele stress). By facing these problems, Biologists compelled to search solution for the homeostasis of soil contents, purification of water and cleaning of environment. Due to biological researchers, a green technology aroused in the shape of phyto remediation, that using of variety hyper accumulator plants to reduce the level of salinity and metals in the polluted and affected soil. The plants having the ability to continue their physiological activities under salt stress condition are suggested for exploiting tolerant cultivars of coastal area, because salt minimize plant growth, alter nutritional balance, specific chemical ionic reactions and homeostasis of plants,15 Which destroy the nature of membrane and produce activated oxygen and cause metal toxicity. Salinity is a vast field of abiotic stress that restrict plant growth and its ecological distribution all over the world especially in Pakistan and Egypt16,17 and18 Cellular metabolisms like growth, photosynthesis, protein and lipid metabolism of plant are severely affected by hyper penetration of soluble salts in arid and semiarid soil region.19 Salt stress can affect growth in plants because of osmotic effect of salt is closely identical to water stress of drought. Secondly when the salt enters to plant causing toxicity in older transpired leaves and ultimately shows premature death. For phyto remediation, Hemerocallis and Dodneae plants having good potentiality to extract and translocase salts and metals from enriched saline metal soils into harvestable parts.
Media (Soil) preparation and plants Transformation
Soil was collected from the herbarium of the University of Malakand, dried in sun light then grinded into fine powdered form and poured into clay pots (3kg soil/pot). Water holding capacity (250mL water/kg soil±4), electrical conductivity (814µs±7) and pH (6.7±2) of the soil was measured. Two different plants (Dodonaea viscosa and Hemerocallis fulva,) were used during the experiment. After germination uniform size plantlets (2 cm roots and 3cm shoot) were selected for the experiment.
Treatments given to plants during the experiment
In the whole experiment Cadmium acetate dehydrate (Cd) solution were added to plants in three different concentrations (50,100 and 150ppm) along with sodium chloride salt in the manner of three different concentrations (1000, 3000 and 6000ppm). Whole experiment was carried out in complete randomised design (CRD) in the manner of three replicates and one control under natural light/dark conditions with temperature 30/25 0C. The following treatments and control (Table 1) were used during the experiment.
Treatments |
Denoted |
Treatments |
Denoted |
Growth media Soil only |
C |
100ppm Cd+1000ppm NaCl |
T4 |
50ppm Cd |
C1 |
100ppm Cd+3000ppm NaCl |
T5 |
100ppm Cd |
C2 |
100ppm Cd+6000ppm NaCl |
T6 |
150ppm Cd |
C3 |
150ppm Cd+1000ppm NaCl |
T7 |
50ppm Cd +1000ppm NaCl |
T1 |
150ppm Cd+3000ppm NaCl |
T8 |
50ppm Cd+3000ppm NaCl |
T2 |
150ppm Cd+6000ppm NaCl |
T9 |
50ppm Cd+6000ppm NaCl |
T3 |
||
Table 1 Application of Cd and NaCl during experiments. C for control is compared with all treatments to find out the effect of Cd alone and in combinations with salt (NaCl) on plant growth. While C1, C2 and C3 are compared with all other treatments for NaCl effect on Cd phyto accumulation
Measurement of plant’s Parameters
To measure the plant parts parameters, the experimental plants in pots were harvested after two month and the length of their parts, roots, stems and leaves was measured through scale. For fresh biomass the plants were separated into roots, stem and leaves) and weighed through physical balance. Each part was packed in envelope and labelled. The samples were kept in oven for dryness at 80°C for 48hrs. Through mortar and pestle the dried samples were crushed into powdered and packed in small polythene bags.
Analysis of Cd in plant tissues after complete degradation in Acid
0.25g from dried samples were taken in conical flask and dissolved in strong acids (Nitric acid and Sulfuric acid in ratio of 5:1) followed the method of 20, Allen (1974) with minor alteration. The flasks were kept on hot plate for 15minutes at 300°C until the white fumes were come out. The acid dissolved solution was cooled, filtered into plastic bottles and for reaching volume up to 50ml, distal water was added.5 to 10ml was taken from each bottle and examined by atomic absorption spectrophotometer for Cd concentration in central resource lab, Peshawar.
Statistical analysis
SPSS-16 and MS-excel (2010) and graph pad prism to analyse the data for actual value of Cd. The data was subjected to ANOVA and the mean values were compared by using Tukey’s Honestly Significant Difference (HSD) test, at P<0.05.
Effect of Cd on Growth, Biomass and water content of Plants
The effect of different treatments on plants growth is shown in Figure 1. The root, stem and leaf length of Hemerocallis and Dodonaea plants are given in Tables 2, Table 3 respectively. All the plants showed significant decrease in growth, biomass and total water content under different Cd concentrations (50, 100 and 150ppm). This decrease was highly significant at the highest concentration of Cd (150ppm) when the control without Cd (C) was compared with Cd treated plants (C1, C2 and C3) as shown in Tables 2, Table 3 respectively for Hemerocallis and Dodonaea plants. At lower concentrations of Cd was not statistically significant as compared to control C (Table 2). Similarly, the lowest concentration of Cd (50ppm) shows non-significant decrease in all the above growth parameters (except the stem length) of Dodonaea plant as compared to the control C±(Table 3). The results showed a gradual decline in growth parameters in all the plants with increasing Cd concentration.
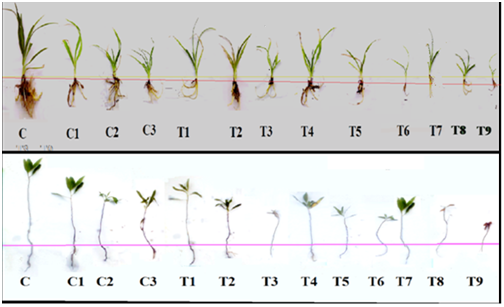
Treatments |
Length cm |
Fresh biomass g |
Dry biomass g |
Total water contents g |
||||||||
|
Root |
Stem |
Leaves |
Root |
Stem |
Leaves |
Root |
Stem |
Leaves |
Root |
Stem |
Leaves |
C |
15±1a |
6.25± 0.25a |
26.3± 1.75a |
10.56± 0.32a |
4.448± 0.61a |
18.5± 0.57 a |
4.226± 0.13a |
1.779± 0.243a |
7.395± 0.227a |
6.3± 0.19a |
2.669± 0.365a |
11.1± 0.34a |
C1 |
12±1b |
4.50± 0.50b |
21.00± 1.75b |
8.65± 1.16b |
3.212± 0.19b |
15.1± 2.03 b |
3.46± 0.46 b |
1.285± 0.07 b |
6.055± 0.813b |
5.2± 0.7b |
1.927± 0.116b |
9.08± 1.22b |
C2 |
11.75± 0.75b |
4.00± 0.05bc |
20.6± 1.31b |
7.435± 0.59 bcd |
2.554± 0.37bcd |
13± 1.04 bcd |
2.974± 0.238 bcd |
1.022± 0.14bcd |
5.204± 0.416bcd |
4.5± 0.36bcd |
1.533± 0.219bcd |
7.81± 0.62bcd |
C3 |
11.25± 1.25 bc |
3.5± 0.5 bcd |
19.7± 2.19bc |
6.373± 0.18 cde |
1.974± 0.01def |
11.2± 0.32 cde |
2.549± 0.074 cde |
0.79± 0.003def |
4.461± 0.129cde |
3.8± 0.11cde |
1.184± 0.004def |
6.69± 0.19cde |
T1 |
11.5±1 b |
4.5± 0.5 b |
20.1± 1.75b |
7.802± 0.18 bc |
3.045± 0 bc |
13.7± 0.32 bc |
3.121± 0.073 bc |
1.218± 0.001bc |
5.461± 0.128 bc |
4.7± 0.11bc |
1.827± 0.002bc |
8.19± 0.19bc |
T2 |
10.5± 0.5bcd |
3.75± 0.25bc |
18.4± 0.88bcd |
6.116± 0.34de |
2.18± 0.08cde |
10.7± 0.59de |
2.447± 0.134de |
0.872± 0.031cde |
4.281± 0.235de |
3.7±0.2 de |
1.308± 0.047cde |
6.42± 0.35de |
T3 |
8.25± 0.75d |
3.25± 0.25cd |
14.4± 1.31d |
5.71±0.2 e |
2.297± 0.46bcd |
9.99± 0.34e |
2.284± 0.079e |
0.919± 0.184bcd |
3.997± 0.138e |
3.4± 0.12e |
1.378± 0.276bcd |
6±0.21e |
T4 |
11.75± 1.25b |
3.6 ± 0.4bcd |
20.6± 2.19b |
6.213± 0.17cde |
1.914± 0.52def |
10.9 ± 0.31cde |
2.485 ± 0.07cde |
0.765 ± 0.208def |
4.349 ± 0.122cde |
3.7 ± 0.1 cde |
1.148 ± 0.312def |
6.52 ± 0.18cde |
T5 |
9.5± 0.5 bcd |
3.2 ± 0.2cd |
16.6± 0.88 bcd |
4.049± 0.81f |
1.366± 0.28efg |
7.09 ± 1.41f |
1.62 ± 0.322f |
0.546 ± 0.114efg |
2.834± 0.564f |
2.4 ± 0.48f |
0.819 ± 0.171efg |
4.25 ± 0.85f |
T6 |
8.75± 1.25cd |
2.5 ± 0.5de |
15.3± 2.19cd |
3.79± 0.14fg |
1.076± 0.1fg |
6.63 ± 0.25fg |
1.516 ± 0.058fg |
0.431 ± 0.042fg |
2.653 ± 0.101fg |
2.3 ± 0.09fg |
0.646 ± 0.062fg |
3.98 ± 0.15fg |
T7 |
12±1b |
3.5 ± 0.5bcd |
21±1.75 b |
6.085± 0.62de |
1.767± 0.22defg |
10.6 ± 1.09de |
2.434 ± 0.25de |
0.707 ± 0.087defg |
4.259 ± 0.437de |
3.7 ± 0.37de |
1.06 ± 0.131defg |
6.39 ± 0.66de |
T8 |
8.5± 0d |
2 ± 0e |
14.9±0d |
3.852± 0.84fg |
0.906± 0.2g |
6.74 ± 1.47fg |
1.541 ± 0.335fg |
0.363 ± 0.079g |
2.697 ± 0.586fg |
2.3± 0.5 fg |
0.544 ± 0.118g |
4.04 ± 0.88fg |
T9 |
4.25± 0.75e |
1.45± 0.05e |
7.44± 1.31e |
2.414± 0.1g |
0.863± 0.22g |
4.22 ± 0.18g |
0.965 ± 0.041g |
0.345 ± 0.086g |
1.689 ± 0.072g |
1.4 ± 0.06g |
0.518 ± 0.13g |
2.53 ± 0.11g |
Table 2 Effect on Hemerocallis plant. C (Soil without Cd and NaCl addition), C1, C2, C3 (50, 100, 150ppm Cd in Soil), T1, T2, T3 (1000, 3000, 6000ppm NaCl+50ppm Cd with each NaCl concentration), T4, T5, T6 (1000, 3000, 6000ppm NaCl+100ppm Cd), T7, T8, T9 (1000, 3000, 6000ppm NaCl+150ppm Cd).±SD denote Standard deviation and different letters show the significant difference among different treatments for a specific parameter
Combine Effect of Cd and Salt (NaCl) on Plant Growth and biomass
The higher concentrations (3000 and 6000ppm) of NaCl salt in combination with Cd significantly decreased the growth, biomass and total water content of both Hemerocallis (Table 2) and Dodonaea (Table 3) plants when C1 (50ppm Cd in Soil) was compared with T2 (50ppm Cd+3000ppm NaCl in Soil) and T3 (50ppm Cd+6000ppm NaCl in Soil). Similarly, when C2 (100ppm Cd in Soil) was compared with T5 (100ppm Cd+3000ppm NaCl in Soil) and T6 (100ppm Cd+6000ppm NaCl in Soil), and C3 (150ppm Cd in Soil) when compared with T8 (150ppm Cd+3000ppm NaCl in Soil) and T9 (150ppm Cd+6000ppm NaCl in Soil) given in Tables 2 & 3. The lower concentration of NaCl (1000ppm NaCl in Soil) in combination with Cd (T1, T4 and T7) showed no significant difference in all the growth parameters when compared C1, C2 and C3 respectively. The highest significant decrease in all the above growth parameters for Hemerocallis plant was recorded for the treatment T9 (150ppm Cd+6000ppm NaCl) as compared to control C. Dodonaea plant showed decrease in plant growth (root and shoot length) and biomass (fresh and dry) with increasing salts (NaCl) concentration. This decrease was significant only at higher salt concentrations (3000 and 6000ppm) when T2, T3 was compared with C1 and T5, T6 was compared with C2, and T8, T9 was compared with C3 (Table 3). The highest significant decrease in all the growth parameters was recorded in the treatment T9 as compared to control C.
Treatments |
Length (cm)±SD |
Fresh biomass (g)±SD |
Dry biomass (g)±SD |
Total water contents (g)±SD |
|||||||||
|
Root |
Stem |
Leaves |
Root |
Stem |
Leaves |
Root |
Stem |
Leaves |
Root |
Stem |
Leaves |
|
C |
25.00± 1.00a |
35.00± 1.00 a |
9.00± 1.00a |
1.98± 0.07a |
2.79± 0.29a |
0.72± 0.13a |
0.79± 0.03a |
1.12± 0.12a |
0.28± 0.05a |
1.19± 0.04a |
1.67± 0.17a |
0.43±0.08a |
|
C1 |
22.50± 0.50ab |
29.50± 0.50 b |
7.50± 1.50ab |
1.79± 0.04ab |
2.35± 0.06 ab |
0.60± 0.14ab |
0.72± 0.02ab |
0.94± 0.03ab |
0.24± 0.05ab |
1.0± 0.02ab |
1.41± 0.03ab |
0.36± 0.08ab |
|
C2 |
21.50± 2.50abc |
22.50± 2.50 c |
5.00± 0.01bcde |
1.54± 0.24bc |
1.61± 0.24cdef |
0.35± 0.01cdef |
0.61± 0.1 bc |
0.64± 0.10cde |
0.14± 0.01cdef |
0.92± 0.14bc |
0.96± 0.14cde |
0.21± 0.0cdef |
|
C3 |
19.50± 0.50bc |
21.00± 1.00c |
4.50± 0.50cde |
1.52± 0.22 bc |
1.63 ± 0.27cdef |
0.34± 0.01def |
0.61± 0.09bc |
0.66± 0.11cd |
0.13± 0.01def |
0.91± 0.13bc |
0.98± 0.16cd |
0.20 ±0.0def |
|
T1 |
21.00± 1.00abc |
29.00± 1.00b |
7.50± 1.50ab |
1.6 ± 0.20ab |
2.28± 0.31ab |
0.57± 0.02abc |
0.66± 0.08ab |
0.91± 0.13ab |
0.23± 0.01abc |
0.98± 0.12ab |
1.36± 0.18ab |
0.34±0.01abc |
|
T2 |
14.50± 0.50de |
15.00± 1.00d |
4.00± 0.01de |
1.43± 0.14bc |
1.47± 0.10cdef |
0.39± 0.05bcdef |
0.57± 0.06bc |
0.59± 0.04cdef |
0.16± 0.02bcdef |
0.85± 0.08bc |
0.88± 0.06cdef |
0.23± 0.03bcdef |
|
T3 |
12.50± 2.50ef |
13.00± 3.00d |
3.85± 0.35de |
1.17± 0.14c |
1.21± 0.18def |
0.36± 0.06cdef |
0.47± 0.05c |
0.48± 0.07def |
0.15± 0.02cdef |
0.70± 0.08c |
0.72± 0.10def |
0.22± 0.03cdef |
|
T4 |
18.00± 2.00cd |
22.52± 0.50c |
7.00± 2.00abc |
1.46± 0.11bc |
1.83± 0.10bc |
0.56± 0.14abcd |
0.58± 0.04bc |
0.73± 0.04bc |
0.22± 0.05abcd |
0.87 ± 0.06bc |
1.09± 0.06 bc |
0.33±0.08 abcd |
|
T5 |
9.50± 1.50fg |
12.50± 2.50d |
4.00± 0.01de |
0.73± 0.01d |
0.90± 0.06fg |
0.31± 0.04ef |
0.29± 0.01d |
0.40 ± 0.02fg |
0.13± 0.01ef |
0.43 ± 0.02d |
0.54± 0.02fg |
0.18±0.03ef |
|
T6 |
8.50± 0.50fg |
11.50± 0.50de |
4.03± 0.07de |
0.71± 0.01d |
0.95 ±0.01fg |
0.33± 0.01ef |
0.28± 0.01d |
0.38± 0.01fg |
0.13± 0.01ef |
0.42 ± 0.07d |
0.57 ± 0.01fg |
0.2±0.01 ef |
|
T7 |
14.50± 1.50de |
22.50± 2.50 c |
6.50± 0.50abcd |
1.17± 0.19c |
1.81± 0.31bc |
0.51± 0.01abcde |
0.47± 0.08c |
0.73± 0.13bc |
0.20± 0.01abcde |
0.70 ± 0.11c |
1.08± 0.18 bc |
0.31± 0.01 abcde |
|
T8 |
7.50± 0.50g |
12.00± 2.00de |
3.65± 0.65ef |
0.65± 0.07d |
1.03± 0.13efg |
0.30± 0.06ef |
0.26± 0.03d |
0.41± 0.05efg |
0.12± 0.02ef |
0.39± 0.04d |
0.61± 0.08efg |
0.04ef |
|
T9 |
7.00± 1.00g |
7.50± 0.50e |
3.10± 0.10ef |
0.58± 0.09d |
0.61± 0.03g |
0.25± 0.01f |
0.23± 0.04d |
0.25± 0.01g |
0.10 ± 0.01f |
0.34± 0.05d |
0.37± 0.02g |
0.15±0.01f |
|
Table 3 Dodonaea plant growth. C (Soil without Cd and NaCl addition), C1, C2, C3 (50, 100, 150ppm Cd in Soil), T1, T2, T3 (1000, 3000, 6000ppm NaCl+50ppm Cd with each NaCl concentration), T4, T5, T6 (1000, 3000, 6000ppm NaCl+100ppm Cd), T7, T8, T9 (1000, 3000, 6000ppm NaCl+150ppm Cd).±SD denote Standard deviation and different letters show the significant difference among different treatments for a specific parameter.
Cadmium Concentration and Accumulation in Plants
Hemerocallis plant showed a significant increase in tissues (Root, Stem and Leaves) Cd concentration with increasing Cd concentration (50, 100 and 150ppm) in soil, when compared C1, C2 and C3 in Table 3 (A). Similarly, the total Cd accumulation in different parts of the plant also increased as the Cd concentration in soil was increased, but this increase was statistically not significant. Salt (NaCl) showed positive and significant effect on Cd concentration and accumulation in various parts of the plant (Table 4). Increasing Cd and sodium salt concentration in the soil increased the Cd concentration in different parts of the plant and thus the highest significant Cd concentration (Root “25.40±2.30ppm”, Stem “46±2.86ppm” and leaf “51±3.00ppm”) was recorded for the treatment T9 (150ppm Cd+6000ppm NaCl).
The highest Cd accumulation (mg/DBM) in root (0.02±0.009 mg/DBM), stem (0.016±0.003 mg/DBM) and entire plant (0.127±0.04mg/DBM) was observed in treatment T9, while in leaves (0.104 ±0.0 mg/DBM) it was observed in T4 (100ppm Cd, without addition of NaCl salt in soil). Increasing Cd concentration in soil increased the Cd accumulation percentage in stem while decreased this percentage in roots and leaves when compared C1, C2 with C3 (Table 4). The highest Cd percentage in roots (25.80±1.18%) was recorded for treatment T7 (150ppm Cd+1000ppm NaCl in Soil), in stem (14.27±3.80%) for C1 (50ppm Cd in Soil) and in leave (72.26±1.56%) for T8 (150ppm Cd and 3000ppm NaCl in Soil). The treatment T6 showed the highest translocation factors 2.21 root-stem and 2.29 root- leaves) and bioaccumulation factor (0.19) as shown in Table 4.
Treatments |
Cd conc.(ppm) |
Cd(mg/DBM) |
Entire plant Cd accumulation(mg/DBM) |
Cd accumulation% |
Translocation Factor |
Bio-concentration factor |
||||||||
|
Roots |
Stem |
Leaves |
R |
S |
L |
|
R |
S |
L |
Root- stem |
Root-leave |
|
|
C1 |
23.00± 1.75bcd |
34.80± 0.38cd |
32.00± 1.00de |
0.08± 0.021a |
0.044± 0.003a |
0.194 ±0a |
0.319±0.06a |
24.9±2 bc |
14.27± 3.80a |
60.79± 1.80e |
1.52 |
1.4 |
0.59 |
|
C2 |
26.00± 2.48ab |
40.00± 0.54bc |
43.20± 1.00bc |
0.08± 0.011ab |
0.041± 0.002ab |
0.225 ±0a |
0.343±0.04a |
22.6± 1.87bcde |
11.87± 2.75abc |
65.57± 1.41bcde |
1.54 |
1.66 |
0.37 |
|
C3 |
32.00± 0.94a |
43.40± 2.38ab |
48.00± 2.00ab |
0.08± 0.007a |
0.0343± 0.007b |
0.214 ±0a |
0.33±0.04a |
24.7± 0.27bc |
10.39± 0.79abc |
64.89± 0.53cde |
1.36 |
1.5 |
0.28 |
|
T1 |
12.00± 1.60e |
11.40± 1.28g |
17.40± 2.00h |
0.04± 0.003cd |
0.0139± 0.001cd |
0.095 ±0b |
0.146±0.01bc |
25.6± 0.27b |
9.47± 0.26abc |
64.87± 0.38cde |
0.95 |
1.45 |
0.3 |
|
T2 |
13.00± 0.88e |
14.20± 1.42g |
24.80± 2.00efg |
0.03± 0.003cd |
0.0124± 0.002cd |
0.106 ±0b |
0.151±0.02bc |
21± 0.83bcde |
8.30± 0.18bc |
70.68± 0.78ab |
1.11 |
1.93 |
0.4 |
|
T3 |
16.80± 2.72de |
16.00 ± 1.00g |
30.40± 3.00def |
0.04± 0.007cd |
0.0146± 0.001cd |
0.121 ±0b |
0.174±0.03bc |
21.7± 1.43bcde |
8.33± 0.72bc |
69.92± 0.72abc |
0.98 |
1.91 |
0.49 |
|
T4 |
16.40± 4.86de |
24.40± 2.28f |
24.00± 1.00fgh |
0.04 ±0.01cd |
0.0186± 0.003cd |
0.104 ±0b |
0.164±0.01 bc |
24.5± 4.44bcd |
11.46± 1.10abc |
64.02± 4.27de |
1.58 |
1.55 |
0.22 |
|
T5 |
17.60± 5.28 cde |
36.20± 1.34cd |
37.40± 2.00cd |
0.03± 0.012d |
0.0197± 0.005c |
0.106 ±0b |
0.154± 0.02 bc |
18.4± 4.97de |
12.82± 3.23ab |
68.72± 2.94abcd |
2.07 |
2.13 |
0.31 |
|
T6 |
17.20± 1.98de |
38.00± 1.36bc |
39.40 ±3.00c |
0.03± 0.004d |
0.0164± 0.004cd |
0.105 ±0b |
0.147± 0.03 bc |
17.8± 0.74e |
11.13± 0.51abc |
71.06± 0.25a |
2.21 |
2.29 |
0.32 |
|
T7 |
22.20± 0.38bcd |
27.00± 0.50ef |
32.00± 4.00de |
0.05± 0.001bc |
0.0191± 0.002c |
0.137 ±0b |
0.21± 0.02b |
25.8± 1.18b |
9.27± 0.66abc |
64.97± 1.66cde |
1.22 |
1.44 |
0.19 |
|
T8 |
22.00± 1.84bcd |
30.60± 0.50de |
43.40± 2.00bc |
0.03± 0.008cd |
0.0112± 0.002cd |
0.118 ±0b |
0.163± 0.03bc |
20.9± 0.72bcde |
6.84± 2.27c |
72.26± 1.56a |
1.39 |
1.98 |
0.23 |
|
T9 |
25.40± 2.30abc |
46.00± 2.86a |
51.00± 3.00a |
0.02 ±0.009 d |
0.016± 0.003cd |
0.086 ±0b |
0.127±0.04 cd |
19.4± 0.56cde |
12.44± 0.10ab |
68.19± 0.66abcd |
1.81 |
2.01 |
0.28 |
|
Table 4 Cadmium concentration and accumulation by various parts of Hemerocallis grown in soil having different concentrations of NaCl and cadmium. C1, C2, C3 (50, 100, 150ppm Cd in Soil), T1, T2, T3 (1000, 3000, 6000ppm NaCl+50ppm Cd with each NaCl concentration), T4, T5, T6 (1000, 3000, 6000ppm NaCl+100ppm Cd), T7, T8, T9 (1000, 3000, 6000ppm NaCl+150ppm Cd) ±SD denote Standard deviation and different letters show the significant difference among different treatments for a specific parameter. R, Roots; S, stem; L, Leaves
Table 5 presents the Cd concentration and accumulation in Dodoneae plant. The highest Cd concentration in roots (32.00± 0.94ppm) of the plant was found in C3 (150ppm Cd only) while in stem (46.00±2.86ppm) and leaves (51.00±3.00ppm) it was recorded for the treatment T9 (150ppm Cd+6000ppm NaCl). Increasing the salt (NaCl) concentration in soil increased the Cd concentration in different parts of the plant (Table 5). The plant accumulated more than 60 % of its Cd in leaves in all treatments. The highest Cd translocation factor (2.21 roots to stem and 2.29 root to leaves) was recorded for the treatment T6 (100ppm Cd+6000ppm NaCl in Soil). The bio concentration factor of the Hemerocallis plant was much less than one (1) for all treatments (Table 5).
The highest significant Cd concentration in roots (74.8±2.86 &78.4±1.36ppm) and stem (62.2±1.58 &64.4±0.9ppm) of Dodonaea viscosa plant was observed in C2 (100ppm Cd in Soil) and C3 (200ppm Cd in Soil) in Table 3 (C). The stem showed significantly high Cd concentration (62.00±1.54 & 63.4±1.58ppm) in treatments T2 (50ppm Cd+3000ppm NaCl in Soil) and T3 (50ppm Cd+6000ppm NaCl in Soil). While leaves possess significantly the highest concentration (98.2±1.56ppm) of Cd in treatment T3 as shown in Table 5. The treatment T1 (50ppm Cd+1000ppm NaCl in Soil) showed the highest root to stem translocation factor (1.22). The root to leaves translocation factor (1.69) was highest in Dodonaea viscosa plant treated with 50ppm Cd+6000ppm NaCl in Soil (T3). Also the Cadmium bio-concentration factor (1.32) was found highest in the treatment T3 (Table 3).
Treatment |
Cd concentration (ppm) ± SD |
Cd accumulation (mg/DBM) ± SD |
Entire plant Cd (mg/DBM) ± SD |
Cd accumulation % |
Translocation Factor (TF) |
Bio-concentration factor (BF) |
|||||||||
|
Root |
Stem |
Leaves |
Root |
Stem |
Leaves |
|
Root |
Stem |
Leaf |
Root to Stem |
Root to Leaves |
|
||
C1 |
57.4± 2.02b |
48.8± 1.11c |
41± 2.5d |
0.04± 0.0002ab |
0.05± 0.0007ab |
0.0098± 0.0026bcd |
0.097± 0.0031a |
42.5± 1.62 |
47.39 ±0.85 |
10.11 ± 2.46 |
0.85 |
0.71 |
1.02 |
||
C2 |
74.8± 2.86a |
62.2± 1.58a |
64.2± 3.54c |
0.05± 0.0018a |
0.04± 0.0014abc |
0.0091± 0.002cd |
0.095± 0.0045ab |
48.28± 1.15 |
42.03 ±0.82 |
9.697 ± 1.79 |
0.83 |
0.86 |
0.68 |
||
C3 |
78.4± 1.36a |
64.4 ±0.9a |
96.6± 1.28ab |
0.05± 0.0076a |
0.04± 0.0063abc |
0.0133± 0.0005ab |
0.103±0.0144a |
46.12 ±0.7 |
40.82 ±0.3 |
13.06 ±0.98 |
0.82 |
1.23 |
0.49 |
||
T1 |
44.4± 1.34cd |
54.2± 1.8b |
43.8± 0.6d |
0.03± 0.0065c |
0.05± 0.0065a |
0.01± 0.0001bcd |
0.089±0.013abc |
32.95 ±0.5 |
55.61 ±1.16 |
11.44 ±1.61 |
1.22 |
0.99 |
0.99 |
||
T2 |
54± 1.02b |
62± 1.54a |
48.4± 1.6d |
0.03± 0.0033bc |
0.04± 0.0061bcd |
0.0077± 0.0006cde |
0.075±0.009bcd |
41.11 ±0.43 |
48.7± 1.29 |
10.19 ±1.69 |
1.15 |
0.9 |
1.14 |
||
T3 |
58± 0.9b |
63.4± 1.58a |
98.2± 1.56a |
0.03± 0.0029c |
0.03± 0.0022cde |
0.0145± 0.0009a |
0.072±0.006cd |
37.4± 0.65 |
42.34 ±1.05 |
20.27 ±0.43 |
1.09 |
1.69 |
1.32 |
||
T4 |
40.6± 0.58d |
45± 0.86d |
48.6± 1.8d |
0.02± 0.0033cd |
0.03± 0.0047cd |
0.0109± 0.0024abc |
0.067±0.0057cde |
35.02 ±1.63 |
48.9 ± 3.28 |
16.08 ±4.90 |
1.11 |
1.2 |
0.44 |
||
T5 |
47.6± 0.86c |
49± 1.02c |
53.6± 3cd |
0.01± 0.0016 de |
0.02± 0.0023fg |
0.0068± 0.0024de |
0.039±0.0024fg |
35.3± 1.45 |
47.55 ± 4.6 |
17.15 ±3.19 |
1.03 |
1.13 |
0.49 |
||
T6 |
54.2± 2.86b |
52.2± 1.04bc |
83.6± 1.34b |
0.02 ± 0.0008de |
0.02± 0.0008efg |
0.0111± 0.0011abc |
0.046±0.0014efg |
33.04±0.78 |
43.09 ±2.81 |
23.87 ±2.52 |
0.96 |
1.54 |
0.58 |
||
T7 |
48.2± 1.58c |
36.4± 1.18e |
24.2± 14.3e |
0.02± 0.0006 cde |
0.03± 0.0005def |
0.005± 0.0018e |
0.054±0.0028def |
41.7± 0.89 |
48.86 ±1.55 |
9.44± 2.34 |
0.76 |
0.5 |
0.26 |
||
T8 |
55± 1.84b |
43.4± 0.92d |
52.4± 1.2cd |
0.01± 0.0041de |
0.02± 0.0049fg |
0.0067± 0.0002de |
0.039±0.0091fg |
36.77 ±0.7 |
46.13 ±0.86 |
17.1± 1.48 |
0.79 |
0.95 |
0.32 |
||
T9 |
55.8± 1.3b |
51± 1.4bc |
64.6± 1.22c |
0.01± 0.0013e |
0.01± 0.0026g |
0.0066± 0.0013de |
0.032±0.0009g |
40.05 ±3.34 |
39.35 ±6.79 |
20.6± 3.45 |
0.91 |
1.16 |
0.37 |
|
|
Table 5 Cadmium concentration and accumulation by various parts of Dodonaea viscosa grown in soil having different concentration s of salt and cadmium.C1, C2, C3 (50, 100, 150ppm Cd in Soil), T1, T2, T3 (1000, 3000, 6000ppm NaCl+50ppm Cd with each NaCl concentration), T4, T5, T6 (1000, 3000, 6000ppm NaCl+100ppm Cd), T7, T8, T9 (1000, 3000, 6000ppm NaCl+150ppm Cd).±SD denote Standard deviation and different letters show the significant difference among different treatments for a specific parameter.
Correlation between plant Cd concentration and dry biomass
Figure 2 shows correlations between dry biomass of different parts (root, stem and leaves) of Dodoneae and Hemerocallis plant species with Cd concentration. Negative correlation found between the dry biomass and Cd concentration in the root, stem and leaves of Hemerocallis plant while the roots of Dodonaea plant possessed a week positive correlation between dry biomass and Cd concentration but negative correlation present in its stem and leaves. From the above results it is stated that increasing of Cd concentrations automatically decreases plant dry biomasses but certain plants showed a little tolerance to such physiological stress conditions.
Cadmium contaminated soil decreases the plant growth, and it could be the negative effect of cadmium on uptake of nutrient and their distribution in the plant cells.21 Its accumulation in plants cell may negatively affect the growth and development of a plant by causing a decrease in the enzymatic activities22 and23 disturb respiration, photosynthesis,24 stomatal closure25 and reduction of nutrient uptake26 The present result showed a decrease in plant growth and biomass due to Cd toxicity. Similar effects of Cd were reported by various investigators on different plants such as on Cucumus sativus27 Lemna polyrrhiza28 and on Glycyrrhiza uralensis.29 Cadmium may affect the root elongation by reducing water and nutrient absorption, decreasing the transpiration rate and consequently decreasing growth rate.s
Salinity in soil and water produces stress condition for plants and may lead to reduction in growth and biomass of a plant.31 It affects plant in three ways, i.e. by decreasing its water potential, ionic imbalance or disturbances in ion homeostasis and its toxicity. Salinity cause physiological drought condition in plants and causes both osmotic as well as ionic stress, thus induce a reduction in growth.32 The suppression of growth is directly related to the total concentration of soluble salts.33 In current experiment salt (NaCl) showed an increasing effect on Cd absorption and accumulation within plant tissues. This increase in Cd content of plant might be due to two mechanismsi.e. exchange of metals from sorption sites in soil by the cationic component and formation of stable metal complexes with the chloride anion.34 Addition of NaCl increased Cd concentration in the soil solution and accumulation in the leaf of Swiss chard.35 It demonstrate that bioavailability of Cd is enhanced under saline conditions. Human-induced salinization and trace element contamination are widespread and increasing rapidly. Phyto-accumulation, as the crucial entry pathway for bio-toxic Cd into the human food stuffs, correlates positively with rhizosphere salinity. Organic matter decreases the bioavailable Cd2+ pool and therefore restricts its Phytoextraction. Sodium salt (NaCl) showed reduction in plant growth and biomass compared to other treatments which might be due to its negative effect on production of endogenous plant growth regulators.36
In the present result, Cd significantly reduced the plant growth, total water content (TWC) and biomass, the same result have been presented by Rubio et al.,37 who reported that plant growth was reduced by Cd uptake and its distribution within cells. According to,38 Cd affects plant growth by damaging membrane permeability and elongation of cell. . Current result showed that NaCl increased uptake of Cd at low concentration up to certain level while maximum amount of NaCl salt did not increase the Cd Phytoextraction. Similar results were found in the work of,30 where sodium salt enhanced Phytoextraction of Cd in optimum condition and cause toxicity to plants that ultimately affected the growth parameter.
In this result specially in hydroponic condition growth parameter were reduced gradually with increase of sodium salt, because salt enhanced the translocation factor of Cd.39 stated that sodium chloride is a biological dilution and improved the Cd concentration with increasing sodium salt concentration.40 suggest that increasing salinity increases cadmium uptake and the reduction of growth has direct proportion to the sodium salt concentration.41 Salt (NaCl) addition to growth media showed an increasing effect on the Cd concentration in different parts of the plant. Cd concentration was enhanced by the gradual increase of salinity.42 Salinity enhanced the chloro-complex with Cd which may lead to increase the translocation of Cd in the cell.42 A Similar increase in Cd concentration in relation with the increase in the NaCl concentration in soil has been reported in potato and sunflower.43
The Cd accumulation increased with the increase of salinity and maximum concentrations were reported in plant roots due to Cd elevation through salt.44 These results are in general agreement with previous studies in which Tamarix ramosissima showed a marked diminution in growth in response to salinity but no diminution in photosynthesis over a salinity gradient from 0 to 200mM NaCl and it was concluded that growth was negatively affected by salinity due to diversion of energy for increased respiration and salt pumping.45 Phyto remediation is a right choice which is applicable to multi-contamination. Laboratory and field trials have proven successful, but this ideal technique is in all cases dependent on plant growth ability on low-fertility soil. While contaminant concentration has often been proposed as an explanation for plant growth limitation, other factors, commonly occurring in industrial soils, such as salinity, should be considered. In order to achieve the goal, the accumulation of Cd via root uptake at different saline conditions were investigated as there is notable evidence that salinity is a key factor in the translocation of metals from roots to the aerial parts of the plant.46
Dodoneae plants grown in soil as well as in acidic and sodic soil too but show tolerance and were found as Cd hyper accumulators, while Hemerocallis plants was not hyper accumulators of Cd. The salt of sodium, although, increased the cadmium concentration in the plant tissues but showed negative effect on plant growth and biomass. Increasing the sodium salt concentration decreased biomass of the plants but showed an increasing effect on the Cd uptake and concentration within different parts of plant. From the results it is clear that the use of saline soil/water containing cadmium metal should be avoided to use for agricultural purposes because of higher absorption of Cd by plants in saline soil/water. It is strongly recommended that plantation and cultivation of Dodoneae plant is very important for phyto extraction of metals in saline soil and conservation of barren rocks.
The higher education commission of Pakistan (HEC) is highly acknowledged for funding this project under the scheme of indigenous PhD fellowship programme of HEC 2012 to 2014.
The author declares no conflict of interest.

©2017 Ahmad, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.