Advances in
eISSN: 2373-6402


Research Article Volume 9 Issue 1
Department of Crop protection, College of Plant Science and Crop production, Federal University of Agriculture Abeokuta, Nigeria
Correspondence: Otusanya MO, Department of Crop protection, College of Plant Science and Crop production, Federal University of Agriculture Abeokuta, Nigeria,
Received: October 20, 2018 | Published: January 4, 2019
Citation: Otusanya MO. Mineral nutrition effect on anthracnose caused by colletotrichum gloeosporioides penz, tuber rot by Botryodiplodia theobromae Pat.and Yield in Dioscorea rotundata var. Iseosi. Adv Plants Agric Res. 2019;9(1):19-25. DOI: 10.15406/apar.2019.09.00405
Mineral nutrition effect on anthracnose caused by Colletotrichum gloeosporioides, tuber rot by Botryodiplodia theobromae and yield in Dioscorea rotundata var. Iseosi was investigated in this study. The field design was RCBD with three treatments of Bounty fertilizer at 0, 0.6 and 1.2 ml l-1 applied at 3 MAP (months after planting0, and three replications. The field plot soil calcium and nitrogen were below the critical determined for optimum yam production in South West Nigeria. Disease incidence was 28% to 39% at 5 MAP, but reduced to 17% to 24% at 6 MAP. Anthracnose severity however was 1, resistant level at 5 MAP and remained at the resistant level of 1 also at 6 MAP. Flavonoid, lignin and tannin content in leaves of the fertilized plants was higher than that of the control at 6 MAP. Leaf calcium content of 1582mg/100g dm (0.6ml-1 treatment) was higher than the control (944mg/100g dm) also at 6 MAP. Six leaf minerals namely N, P, K, Mg, S and Fe were higher in the Bounty-fertilized plants than the control. Leaf Ca correlated significantly with lignin, and leaf N, P, Fe with leaf tannin and tannin with lignin. Calcium levels across the three treatments, high N, P, K, S, Fe in bounty-fertilized plants and increased levels of lignin, tannin and flavonoid (by positive correlations) is responsible for the resistant severity level in variety Iseosi. Leaf calcium range in this study of 944 to 1039.7 mg/100g dm is appropriate in anthracnose control in variety Iseosi and could be maintained to control or to determine anthracnose resistance in white guinea yam varieties in Nigeria. Bounty fertilizer increased tuber phenol over the control, while control plants tuber phenol was in turn higher than that of farmers’ plot tubers. Tuber calcium range of 11.81 to 13.41mg/100g dm was comparable across treatments. Low infection of 1.3% and no weight loss after incubation of tubers with B. theobromae, in this study is lower than reported for reduction of yam tuber infection and weight loss by B. theobromae with calcium fertilization. Yield in tons per hectare in this study is mean value of 22.07 tons per hectare, which is 170% higher than the expected yield of 10 to 15 tons per hectare with 400kg (8 bags) of NPK 15-15-15 per hectare (60 kg N, 60 kg P2O5, 60 kg K2O ha-1), under continuous cultivation in the forest zone of Nigeria.
Tuber of the Dioscoreaspecies are a staple in the tropical world. Production in Nigeria, the worlds’, largest producer and export is constrained by the field disease anthracnose, caused by Colletotrichum gloeosporioides Penz, and storage losses due to microbial (mainly fungi) infection. Balanced mineral nutrition is being explored for management of these two major constraints with cost effectiveness and less hazard to health and environment. The added nutritional effects of balanced yam nutrition are also a requirement for improved yam production. This study investigated the effect of an 8-mineral component fertilizer called Bounty on Anthracnose disease in Dioscorea,rotundata var, Iseosi (a popular white guinea yam variety in the South West area of Nigeria) as well as infection and weight loss in tubers incubated with Botryodiplodiatheobromae Pat, one of the economic pathogens of stored yams. Improvement of proximate content and minerals of the tubers is compared also with tubers of the variety sourced from farmers’ plots where production is based on natural replenishment after two to three years fallow.
Soil sampling
The field plot which had been mono cropped to Dioscorea species with only Calcium nitrate fertilizer application in two previous consecutive years (2015 and 2016) was cleared of vegetation, manually with hoes and machetes. Old bamboo stake were burnt on the field plot. An area of 14m, by 9.5m was marked out and divided into 3 replicates. Twenty (20) core soil samples were collected per replicate, in a zig-zag pattern, at 20 cm depth, with a soil anger in slanting position (to lift upper and lower soil).1 Collection was in new labelled polythene bags. Soil samples were air-dried, sieved with a 2 mm sieve, then bulked according to replicates. Soil pH was determined using a glass electrode pH metre in a 1:2 soil: distilled water ratio. Soil analysis for calcium, magnesium, nitrogen, phosphorus and potassium was done using standard methods,1 at the Soil Science Laboratory of COPLANT (College of Plant Science and Crop Production).Federal University of Agriculture, Abeokuta (FUNAAB), Ogun State, Nigeria.
Field layout
The plot used is located at the Teaching and Research Farms of the Directorate of University Farms (DUFARMS) Fed. Univ. of Agric. (FUNAAB), Abeokuta. The plot size was 14m by 9.5m. The design of the plot was RCBD (Randomized Complete block design) with 3 treatments and 3 replicates. The treatments were Bounty fertilizer soil amendment at the rates 0ml Ɩ-1 (control), 0.6ml Ɩ-1 and 1.2ml Ɩ-1. The mounds were 1m by 1m and 80 cm high. Inter-row and intra-row spacing were 1m and 0.5 m respectively. One metre (1m) field border was maintained, all round he plot. One 2m length stake was established per mound.
Planting and fertilization
Tubers of average size 0.6kg were planted per mound at the onset of steady rains (first week of April). Bounty fertilizer was applied at 3MAP (months after planting).2 It was prepared with distilled de-ionized water at the rate 0ml Ɩ-1 (control) 0.6ml Ɩ-1 and 1.2ml Ɩ-1.Grooves of about 20 cm depth were made at 25cm radius around the roots of the plant on each mound,2 for the fertilizer. Five hundred and fifty millibitres (550ml) of the fertilizer liquid was poured per mound with a plastic bowl, evenly in the groove and it was closed up well with soil.
Assessment of anthracnose incidence and severity
Disease Incidence was determined by counting number of plants with anthracnose symptoms per replicate. Percent incidence was calculated with the formula:
Anthracnose Severity was determined with a Yam Anthracnose Disease Severity Assessment Scale of 1, (resistant) to 5 (very susceptible) as shown in (Figure 1-3).3 Incidence and Severity were assessed at 5 MAP and 6 MAP.
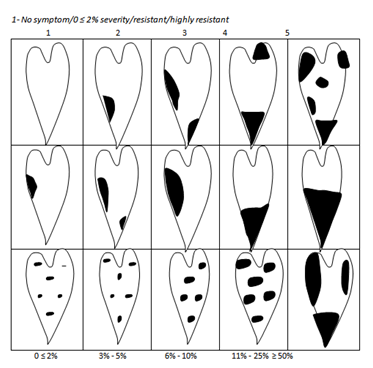
Figure 1 Yam leaf anthracnose disease assessment diagram + lesions on petiole and ste
2 - Necrotic spots 1.00 mm – 2.00 mm/3-5% severity/moderately resistant (tolerant) 3 - Necrotic spots 2.01 mm – 3.00 mm/6-10% severity/moderately susceptible 4 - Necrotic spots 3.01 mm – 4.00 mm/11-25% severity/susceptible 5 - Necrotic spots > 4.00 mm/50% severity/very susceptible Modified from Green (1994)

Figure 2 Leaf anthracnose assessment
Petiole 1/5 x 100 = 20% , Leaf BLADE 1/10 = 5% , 2/10 = 10% ETC. 10 Leaves UPPER PORTION (young = tender/lighter texture) 10 Leaves MIDDLE PORTION (maturing = green/expanded/big) 10 Leaves LOWER PORTION (fully expanded + well-formed Cuticle/texture leathery)
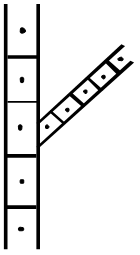
Figure 3 Vine and Petiole anthracnose assessment
5 Sections, 5 Sections -2, Vines only TDA Vine only, TDR Vines only, TDA (local) Estimated % area scored per Vine/Petiole (5 sections)
Leaf sampling for analysis at 6 MAP
Leaf sampling for analysis was carried out by tagging/labelling plants along a diagonal transect of the plot, so as to cover treatments randomly. Twelve (12) leaves were collected (with petioles attached) from a tagged plant, four each from the top, middle and lower portion (near the mound) of the plant, into new large labelled paper envelopes early in the morning, 7:00am.Treatmentswere in triplicate. They were transferred to the Crop Protection Laboratory, COPLANT building where they were washed in plastic bowls containing distilled de-ionized water. Drying of the leaves thereafter was in new labelled food paper packs on raised, wooden, netted yam storage structures in the COPLANT screen house, for 2 to 3 days. The dried leaves were then ground to powder with a Saisho 2-speed Warring blender into new labelled polytheylene bags. Analysis of the phytochemicals, lignin, tannin, and flavonoid as well as the minerals calcium, nitrogen phosphorus, potassium, magnesium, iron and sulphur was carried out at the Biotechnology Centre Laboratory FUNAAB, Biological Sciences Tetfund Laboratory FUNAAB, using routine methods of the A.O.A.C.4
Tuber sampling and analysis for proximate content, phenol content and minerals at 6 MAP
Sampling of tubers was along a diagonal transect of the field plot, with random selection of treatments per replicate. Sampling was in triplicate per treatment. Tubers were carefully harvested with smooth wooden spoons. Moving of soil was done so as not to bruise tubers. Harvest of tubers along the diagonal transect was for tubers for analysis and the infection experiment. Harvested tubers were cleared of soil with a soft cloth and transferred to the Crop Protection Laboratory. They were washed in tap water and left to dry on laboratory benches. Thereafter each tuber for analysis was sliced into thin chips with a steel knife on to labelled plastic trays. The chips were transferred to dry (under shade) on top of raised wooden, netted yam storage structures in the COLPLANT Screen house for 2 to 3 days. The dried chips were milled with a hammer and bull mill, as well as a high powdered mill at the Central Workshop of the College of Engineering (COLENG), FUNAAB. Milled Samples were poured into new labelled polythene bags. Analysis for proximate and phenol contents as well as minerals was carried out according to routine methods of the A.O.A.C.4 at the Biotechnology Centre, FUNAAB and also at the Biological Sciences Tetfund Laboratories, College of Biological Sciences, FUNAAB. Proximate analysis was for dry matter, moisture, fat, ash, crude fibre, crude protein and carbohydrate according to percent dry matter. Phenol and the minerals calcium, magnesium, nitrogen phosphorus potassium, Iron and Sulphur were determined in milligrams per 100 g dry matter.
Infection experiment
Triplicate tubers of about 0.8 to 1 kg size, per treatment were dusted free of soil with a soft cloth labelled and weighed with a top loading balance. Inoculation of tubers was done in an Inoculation Hood structure, in the Crop Protection Lab., COPLANT, FUNAAB. Inoculation sites on each tuber was surface–sterilized by swabbing with cotton wool dipped in 80% ethand. Two cork borers of 6mm ad 4mm diameter, scalpel and pair of forceps were sterilized by dipping into 90% methylated spirit and flaming to red hot over a lighted spirit flame bottle. They were then allowed to cool slanted against another cork borer. Holes of 10 to 12mm size were made through the inoculation sites on each tuber with the sterilized 6 mm cork borer, scalpel and forceps. The sterilized 4 mm cork borer was them used to lift a 4mm disc of a 10–day old pure culture of Botryodiplodiatheobromae (on potato dextrose agar) in a 9cm Petridish, into the bored hole in the tuber. The bored tissue was then replaced with sterile forceps and the inoculation site sealed with vaseline (petroleum jelly). Inoculated tubers were transferred into the raised wooden netted yam storage structures in the COLPLANT Screen house for a period of two weeks. After the 2 weeks period, Vaseline was cleared from tubers with a spatula and cotton wool and each tuber weighed again with a top loading field scale. Each tuber was cut thereafter through the inoculation site, perpendicular to the tuber length with a steel knife. Infected tissue was then cut away from both halves with a scalpel on to a pre-weighed open 9cm petri-dish. Infected tissue weight was determined with an electronic balance. Percent weight loss was calculated thus;
Where A and B are weights of the tuber at the beginning and end of the experiment respectively. Infected tissue weight was corrected for weight loss in the tuber with the formula
(Corrected weight of infected tissue) Otusanya and Jeger,1994.
Percent infection was then calculated with formula: % Infection =
Where C and A corrected weight of infected tissue and weight of tuber at the beginning of the experiment respectively.
Yield assessment at 6 MAP
Tuber Harvest from mounds was done with smooth wooden spoons, by carefully moving/pushing soil away from the mound, to locate the tubers, after the above-ground portion had been cut away with a sharp machete. Tubers were twisted gently clockwise to detach from the mother shoot. They were laid to dry on mounds for about 3 hours. They were then cleared of soil, counted and weighed per mound/plant with a top loading field scale. Transfer of yams to the storage structures was with big raffia baskets.
Data analysis
Data obtained were subjected to Analysis of variance, and means were separated with Turkey’s HSD test.
Soil analysis
Soil analysis results indicate soil pH as 7.4, in the experimental site (Table 1). Soil Nitrogen and Calcium were 1.17% and 0.45cmol kg-1 respectively below the critical required for optimum yam production in South West Nigeria.5 However soil phosphorous, and potassium were 44.47mg kg-1 and 0.68cmol kg-1, both of which are above the critical of 25mg kg-1 and 0.40cmol kg-1 respectively for optimum yam production in South West Nigeria.5
pH (in water) |
7.40±0.200 |
Ca (cmolk-1) |
0.45±0.003 |
Mg (cmol k-1) |
0.51±0.004 |
K (cmol k-1) |
0.68±0.004 |
N (%) |
1.1±0.50 |
P (available mg k-1) |
44.47±0.54 |
Table 1 Mean values of Soil properties of the experimental site
Disease incidence and seventy
Anthracnose disease incidence at 5 MAP was 39.11%, 27.78% and 27.78% in the 0, 0.6 and 1.2ml Ɩ-1 Bounty fertilizer treatments respectively as shown in Table 2. Incidence in the control treatment was significantly higher than the fertilized treatments, which were not significantly different from on e another. At 6 MAP, incidence was lower in the 0.6ml Ɩ-1 treatment (16.67%), than the 1.2ml Ɩ-1 (33.33%) and control (27.78%) which was not significantly different from one another (Table 2). Severity score however was 1 for all 3 treatments at 5 MAP, which is the resistant level. Severity of 1 across all treatments did not change at 6MAP (Table 3).
Bounty fertilizer treatment ml l-1 |
Disease incidence (%) at 5MAP |
Disease incidence (%) at 6MAP |
0 |
39.11a |
27.78b |
0.6 |
27.78b |
16.67c |
1.2 |
27.78b |
33.33a |
Table 2 Anthracnose disease incidence in Bounty-fertilized Dioscorea rotundata variety Iseosi at 5MAP and 6MAP
Means in a column followed by the same letter are not significantly different at P=0.05 (Tukey’s Test)
Bounty fertilizer treatment, ml l-1 |
Disease severity at 5 MAP |
Disease severity at 6 MAP |
0 |
1 |
1 |
0.6 |
1 |
1 |
1.2 |
1 |
1 |
Table 3 Anthracnose disease severity score in Bounty-Fertilized Dioscorea rotundata var. Iseosi at 5 MAP and 6 MAP
Leaf phytochemical and mineral content analysis
Lignin content in leaves of the 0.6 ml Ɩ-1 fertilized plants was higher that is (3256.67mg/100gdm) than the control (3120mg/100gdm). Values of lignin for the two fertilizer treatments were not significantly different from one another. Leaf flavonoid was higher in the two fertilizer treatment than the control. The values were 670> 646.67>636.67mg/100gdm (Table 4). Tanning content were significantly higher also in the 2 fertilizer treatments, than in the control, that is 5.12>4.89>3.21mg/100gdm. Calcium was higher in the 0.6ml Ɩ-1 treatment (1582mg/100gdm) than the control (944mg/100gdm), (Table 4), but the 1.2 ml Ɩ-1 (1039.7mg/100g dm) calcium content was similar to the 0.6 ml Ɩ-1 treatment. The six other minerals Mg, Fe, N, P, S and K were significantly higher in the Bounty–fertilized treatments than the control as follows:
Leaf N, 596.33 (1.2ml Ɩ-1)>572.33 (0.6 ml Ɩ-1)>553.50 (control), mg/100gdm
Leaf P, 66.79 (1.2ml Ɩ-1)>66.30 (0.6 ml Ɩ-1)>65.19 (control), mg/100gdm
Leaf K, 68.14 (1.2ml Ɩ-1) <75.72 (0.6 ml Ɩ-1)>60.19 (control), mg/100gdm
Leaf Fe, 0.811 (1.2ml Ɩ-1)>0.778 (0.6 ml Ɩ-1)>0.728 (control), mg/100gdm
Leaf S, 1316.33 (1.2ml Ɩ-1)>1244.33 (0.6 ml Ɩ-1)>1213.5 (control), mg/100gdm
Leaf Mg, 222.87 (1.2ml Ɩ-1)= 267.03 (0.6 ml Ɩ-1)>191.35 (control), mg/100gdm
Fertilizer (Bounty) ml l-1 |
Phytochemicals |
Minerals |
||||||||
Lignin |
Flavonoid |
Tannin |
Calcium |
Magnesium |
Iron |
Nitrogen |
Phosphorus |
sulphur |
Potassium |
|
0 |
3120.00b |
636.67c |
3.21c |
944.0b |
191.35b |
0.728c |
553.50c |
65.19c |
1213.50c |
60.19c |
0.6 |
3256.67a |
646.67b |
5.12a |
1582.0a |
267.03a |
0.778b |
572.33b |
66.30b |
1244.33b |
75.72a |
1.2 |
3183.33ab |
670.00a |
4.89b |
1039.7ab |
222.87a |
0.811a |
596.33a |
66.79a |
1316.33a |
68.14b |
Table 4 Phytochemicals (mg/100gdm) and minerals (mg/100gdm) in leaves of Bounty-fertilized Dioscorea rotundata variety Iseosi at 6 months after planting
Means in a column followed by the same letter are not significantly different at P = 0.05 (Tukey’s Test)
Correlation of phytochemicals, minerals, disease incidence, severity and tuber infection
Only significant correlations at p=0.01/0.05 are presented in Table 5. Leaf calcium correlated positively with lignin (r=0.8472) and magnesium (r =0.7566). Leaf Nitrogen correlated with tannin (r=0.7435), Fe (r= 0.9619), S (r=0.9592) and P (r=0.9362). Leaf Phosphorus correlated with tannin (r=0.9138), Fe (0.9839) and S (r= 0.9009). Leaf Iron correlated with tannin (r =0.8632) and tannin with lignin (r =0.7867). Disease incidence at 5 MAP correlated negatively with tuber infection (r=-0.6874).
|
Ca |
Mg |
N |
P |
K |
S |
Fe |
Flavonoid |
Tannin |
Lignin |
DI at 5 MAP |
DI at 6 MAP |
DSS at 6 MAP |
Tuber % Infection |
Ca |
|
0.7566* |
|
|
|
|
|
|
|
0.8472* |
|
|
|
|
Mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
0.9362* |
|
0.9592* |
0.9619* |
|
0.7435* |
|
|
|
|
|
P |
|
|
|
|
|
0.9009* |
0.9839* |
|
0.9138* |
|
|
|
|
|
K |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S |
|
|
|
|
|
|
0.9228* |
|
|
|
|
|
|
|
Fe |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Flavonoid |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tannins |
|
|
|
|
|
|
0.8632* |
|
|
0.7867* |
|
|
|
|
Lignin |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DI at 5 MAP |
|
|
|
|
|
|
|
|
|
|
|
|
|
-0.6874* |
DI at 6 MAP |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DSS at 6 MAP |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tuber % Infection |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 5 Significant correlations of Anthracnose incidence/severity, leaf anticipins/minerals and tuber infection in D. rotundata var. Iseosi
Significant correlations at P = 0.001 to 0.05
Proximate content and phenol content in tubers
Proximate and phenol content of tubers from the field plot are presented in Table 6, along with these components in tubers of the variety sourced from farmers’ plots. Dry matter of farmers’ plot tubers compared favorably with the control in this study, 87.76 and 85.74% respectively (Table 6) Dry matter than decreased from the 0.60ml Ɩ-1 treatment (82.10%) to the 1.2ml Ɩ-1 treatment (65.03%). Moisture was highest in the 1.2ml Ɩ-1 treatment (34.97%) and was not significantly different in the remaining two treatment (17.90 and 14.26%), which were comparable to these from farmers’ plot (12.24%). Carbohydrate was highest in the 0.6ml Ɩ-1 (99.29% dm), while the other three were similar (79.77, 81.44, and 80.21%dm) (Table 4). Fat content had the range 0.50 to 0.55 % dm and was similar in all treatments. Crude protein had the range 4.81 to 5.58% dm and was similar in all treatments (Table 6) Ash content with the range 2.38 to 2.51% dm was also similar in all treatments. Crude fibre was similar in the 0.6ml Ɩ-1 (11.13% dm), 1.2ml Ɩ-1 (11.38%dm) and farmers plot (11.63% dm). The latter, (farmers’ plot) and the 1.2ml Ɩ-1 crude fibre content were significantly higher than that of the control (10% dm). Phenol content was highest in the 1.2ml Ɩ-1 (81.72mg/100gdm), followed by 0.6ml Ɩ-1 (78.93mg/100dm), then the control (72.17mg/100gdm) and lowest phenol content was in the farmer’s plot tubers (66.53mg/100gdm) as shown in Table 4. Calcium, a mineral is in a column next to phenol, to compare their values. The values of calcium are in the range of 15% to 19% of the corresponding values for phenol (Table 6).
Bounty-fertilizer |
Dry matter |
Moisture |
CHO |
Fat |
Crude fibre |
Crude |
Ash |
Calcium mg/100gdm |
Phenol mg/100gdm |
FPT |
87.76a |
12.24b |
80.21b |
0.53a |
11.63a |
5.16a |
2.50a |
12.53a |
66.53d |
0 |
85.74a |
14.26b |
81.44b |
0.52a |
10.00b |
5.58a |
2.47a |
13.41a |
72.17c |
0.6 |
82.10b |
17.90b |
99.29a |
0.50a |
11.13ab |
4.81a |
2.38a |
13.02a |
78.93b |
1.2 |
65.03c |
34.97a |
79.77b |
0.55a |
11.38a |
5.47a |
2.51a |
11.81a |
81.72 |
Table 6 Proximate composition (% of dry matter), calcium and phenol (mg/100gdm) content in tubers of Bounty-fertilized Dioscorea rotundata variety Iseosi at 6 months after planting
Means in a column followed by a common letter are not significantly different at P = 0.05(Tukey’s test)
Mineral content of tuber
Mineral content of tubers are compared to that of farmers’ plot tubers (FPT) also in Table 7. Calcium with the range 11.81 to 13.41mg/100gdm was not significantly different in the four treatments. Magnesium with the range 24.46 to 25.94mg/100gdm was also similar in all four treatments. Tuber potassium varied significantly as follows:85(1.2ml Ɩ-1)>79.23 (0.6ml Ɩ-1)>66.8(farmers plot=FPT)>59.21 (control). Tuber Nitrogen varied significantly as follow: 786 (1.2ml Ɩ-1)>762 (0.6ml Ɩ-1)>729.67 and 720.33 (control and FPT). Tuber phosphorus varied significantly as follows: 62.08 (1.2 ml Ɩ-1)> 61.44, 61.19 (0.6 ml Ɩ-1, control) > 60.71 (FPT). Tuber sulphur varied significantly as follows: 974.67 (1.2 ml Ɩ-1)> 948 (0.68 ml Ɩ-1) > 916 (control) > 893.67. (Farmer plot). Tuber Iron varied significantly as follows: 0.62 (1.2 ml Ɩ-1) > 0.59 (0.69 ml Ɩ-1) > 0.58 (control) > 0.56 (FPT).
Bounty fertilizer |
Phenol |
Calcium |
Magnesium |
Potassium |
Nitrogen |
Phosphorus |
Sulphur |
Iron |
0 |
72.17c |
13.41a |
24.46a |
59.21d |
729.67c |
61.19b |
916.00c |
0.58c |
0.6 |
78.93b |
13.02a |
24.68a |
79.23b |
762..00b |
61.44b |
948.00b |
0.59b |
1.2 |
81.72a |
11.81a |
25.94a |
85.00a |
786.00a |
62.08a |
974.67a |
0.62a |
FPT - |
66.53d |
12.53a |
25.04a |
66.80c |
720.33c |
60.71c |
893.67d |
0.56d |
Table 7 Phenol and minerals content (mg/100gdm) in tubers of Bounty-fertilized Dioscorea rotundata variety Iseosi at 6 months after planting
Means in a column followed by the same letter are not significantly different at P = 0.05 (Tukey’s Test
FPT, Farmers’ Plot Tubers; FPT, Farmers’ plot tuber
Infection and weight loss in tubers after 2-weeks incubation with botryodiplodiatheobromae
Infection range was 0.96 to 1.48% in the three treatments with no significant difference. There was no weight loss (0%) in any of the inoculated tubers after the 2- week’s incubation, (Table 8).
Bounty fertilizer : |
Infection % |
Weight loss % |
0 |
0.96a |
0 |
0.6 |
1.19a |
0 |
1.2 |
1.48a |
0 |
Table 8 Infection (%) and weight loss (%) in tubers of Bounty-fertilized Dioscorea rotundata variety Iseosi after 2 weeks incubation with Botryodiplodia theobromae
Means in a column followed by the same letter are not significantly different at P = 0.05 (Tukey’s Test)
Yield at 6MAP
Tuber number per plant across the three treatments was 2.42 to 3.69 with no significant difference between them (Table 9). Also tuber weight per plant across the three treatments was 2.09 to 2.36 kg and was not significantly difference in the three treatments. Yield equivalent in tons per hectare for the three treatments are 21.70 tons ha-1 (control), 20.90 tons ha-1 (0.6ml Ɩ-1) and 23.60 tons ha-1 (1.2ml Ɩ-1), (Table 9).
Bounty fertilizer |
Tuber number |
Tuber weight (kg) |
Tuber weight in tons |
|
0 |
2.42a |
2.17a |
21.7 |
|
0.6 |
3.23a |
2.09a |
20.9 |
|
1.2 |
3.69a |
2.36a |
23.6 |
|
Table 9 Yield in Bounty-fertilized Dioscorea rotundata variety Iseosi at 6 months after planting
Means in a column followed by the same letter are not significantly different at P =0.05 (Tukey’s Test)
This Project was carried out in a field plot that had been mono cropped to yours (Dioscorea spp.) for two previous consecutive years (2015, 2016), with application of calcium nitrate by soil amendment. The field plot was used in this study (2017) with the application of Bounty fertilizer which is made up of 8 minerals. The concentration of the minerals in the fertilizer is as follows: Calcium 7% w/w, Nitrogen 15% w/w, Magnesium Oxide 0.5% w/w, Copper 0.5% w/w, Zinc 2% w/w, Iron 2% w/w, Manganese 1% w/w, and Boron 0.0025% w/w. Calcium and Nitrogen content are the highest in Bounty fertilizer and supply of both was justified as these two minerals were below the critical of 0.65cmol kg-1 and 2% required for optimum yam production in South West Nigeria.5
However, phosphorus and potassium in the field plot were above the critical required. Phosphorus and potassium were not supplied from Bounty fertilizer. Anthracnose incidence was about 28% in the fertilized plots at 5 MAP, but was higher (over 39%) in the control plots. All of these had decreased by 6MAP and the lowest in the 0.6ml Ɩ-1Bounty fertilized plots was only 16.67%. Severity however, at 5 MAP was 1, resistant level, across all treatments including the control. The same severity status of 1 (resistant) was maintained across all treatments at 6 MAP. Two calcium nitrate foliar sprays (in the 9 months growth cycle), in D.rotundata TDr 131 had resulted in the severity score of 3, or moderately susceptible at 6 MAP, and an increase in yield in the only report of use of calcium nitrate for anthracnose control.3 The three leaf Phytochemical or phytoanticipins with antimicrobial properties6 were higher in the Bounty-fertilized plants than the control. Leaf calcium was higher in the 0.6 ml Ɩ-1 treatment that the control, but was not significantly different from leaf calcium content in the 1.2 ml Ɩ-1 fertilized plants.
Calcium has been implicated in the resistance to disease in crop plants due to its role in maintaining plasmic membrane stability and structural integrity of plant cell wals.7-9 Leaf Nitrogen, phosphorus potassium, magnesium, iron and sulphur were all higher in the Bounty fertilized plants than the control. Nitrogen and potassium foliar application have reduced Alternaria disease in potato, tomato and cotton.10 In this study leaf Ca correlated significantly with lignin content. Leaf N, P and Fe correlated significantly with tannin content and tannin correlated significantly with lignin. This corroborates the fact that nutrients are involved in the tolerance or resistance mechanisms of the host plant.11
The increased levels of the lignin, tannin and flavonoid by Bounty fertilizer were promoted also by the increased levels of the Nitrogen, Phosphorus and Iron minerals in the leaves of the fertilized plants and may be adduced as the reason for the resistance status (Severity score 1) of variety Iseosi to anthracnose disease. Calcium level (in the leaves) in the control plant was not significantly different from that in the 1.2ml Ɩ-1 Bounty-fertilized plants. This calcium level in the control was supplied from the field plot soil which had 0.45 cmol kg-1 calcium, which is below-critical level for optimum production in Dioscorea in South West Nigeria5 but which is higher than the 0.19 to 2c mol kg-1 in the report where Calcium carbonate soil amendment reduced tuber infection and weight loss by B. theobromae in 2 varieties of Dioscorea.12 The fact that control plants in this study also had anthracnose resistant status indicates that this leaf calcium content may be appropriate to control anthracnose in variety Iseosi and may underscore the need to maintain the leaf calcium level to control/determine anthracnose resistance in other local white guinea yam varieties in Nigeria. Phenol content was higher in the tubers of Bounty–fertilized plants than the control, and the phenol content of control tubers was in turn higher than of farmers’ plot tubers.
Phenol and phenolics have been implicated in resistance to microbial infection in plants.13,14 Weight loss and infection in the tubers after incubation with Botryodiplodiatheobromae in this study were 1.3% (mean, as there were no significant differences) and 0% respectively. This infection is 38% and 77% of infection by B. theobromae in year one and two respectively, in two improved varieties of Dioscorea (D. rotundata TDr 131, D. alata TDa 92-2) in the only report of reduction of tuber infection by calcium fertilizer (Otusanya et al., 2016). In the latter cited report also weight loss was 3.12 and 3.64% in year one and two respectively, but no weight loss (0%) was recorded in 2 weeks in this study. This is thus greater reduction in yam tuber weight loss and infection by B. theobromae with the 8 mineral component fertilizer Bounty compared to Calcium carbonate. Fertilization with Bounty in yam production is recommended for the control of both anthracnose and tuber infection by B. theobromae, in white guinea yam in South West Nigeria. Nutritionally Bounty fertilizer is recommended as nitrogen, potassium, sulphur and iron were higher in Bounty-fertilized tubers compared to farmers’ plot tubers. The negative correlation (r=-0.6874) between disease incidence at 5 MAP and tuber infection may indicate tuber inducement of phenol as a post–inhibition. Yield equivalent in this study is 20.9 to 23.6 tons ha-1 (average of 22.07 tons ha-1) which is over 170% higher than the expected yield of 10 to 15 tons ha-1with 400kg (8 bags) of NPK 15-15-15 per hectare (60kg N, 60kg P205 and 60kg k20 per hectare), under continuous cultivation in the forest zone of Nigeria (ICS-Nigeria).15
None.
The authors declared there is no conflicts of interest.

©2019 Otusanya. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.