Advances in
eISSN: 2373-6402


Research Article Volume 9 Issue 1
1Medicinal Plants and Economic Development (MPED) Research Centre, Department of Botany, University of Fort Hare, South Africa
2Elizade University, Nigeria
Correspondence: Otunola GA, Medicinal Plants and Economic Development (MPED) Research Centre, Department of Botany, University of Fort Hare, Private Bag X1314, Alice 5700, South Africa,, Tel 27745276130
Received: January 07, 2019 | Published: January 22, 2019
Citation: Ayodele AAM, Otunola GA, Afolayan AJ. Micro morphology of Foeniculum Vulgare Mill. (Apiaceae). Adv Plants Agric Res. 2019;9(1):147-151. DOI: 10.15406/apar.2019.09.00427
Background: Foeniculum vulgare Mill. belongs to the family Apiaceae. The general population of Eastern Cape of South Africa make use of this plant for culinary and medicinal purposes. The leaves, stems and seeds of Foeniculum vulgare were accumulated from the shrubbery where it develops in wealth in Alice, Eastern Cape Province of South Africa.
Materials and methods: The leaves, stems and the seeds were fundamentally watched utilizing the Scanning Electron Microscopy (SEM) associated with Energy Dispersive X-beam (EDX) spectroscopy. The structures under study include the stomata types, an outline of epidermal cells and the crystals.
Results: The outcomes indicated measure-up to circulation of stomata on both adaxial and abaxial surfaces of leaf and stem, precious stones were found in plenitude in both surfaces, the X-ray elemental analysis of the leaf, stem and seed of F.vulgare created spectra of micro and macro mineral components; Calcium (Ca), Oxygen (O), Sodium (Na), Aluminium (Al), Silicon (Si), Phosphorus (P), Sulphur (S), Chlorine (Cl), Potassium (K), Iron (Fe), Bromine (Br), Carbon (C), Magnesium (Mg).
Conclusion: The characters observed in this plant could be added to the data obtained from other lines of evidence for the improvement of the delineation, identification, systematic repositioning and roles of Foeniculum vulgare in economic development.
Keywords: Foeniculum vulgare, spices, elemental composition, scanning electron microscope
In general plant studies, the Apiaceae family (Umbelliferae) is one of the biggest plant families on the planet which includes more than 455 genera and 3600-3780 species.1 Members of the family are widely distributed almost all over the world from temperate to subtropical and tropical regions. The family includes some important aromatic, medicinal plants and culinary herbs and spices such as fennel (Foeniculum vulgare Mill.), female ginseng (Angelica sinensis (Oliv.) Diels), asafetida (Ferula assa-foetida L.), cumin (Cuminum cyminum L.), anise (Pimpinella anisum L.), coriander (Coriandrum sativum L.), parsley (Petroselinum crispum (Mill.) Fuss), and carrot (Daucus carrota L.). The general population of Eastern Cape of South Africa make use of this plant for culinary and medicinal purposes.2 There is limited information on the leaves, stems, shoots, flowers and seeds anatomy and micro morphology of Foeniculum vulgare. We, therefore, used this research to evaluate the morphology and essential examination of leaves stems and seeds of Foeniculum vulgare with the aid of Scanning Electron Microscopy (SEM) associated with Energy Dispersive X-beam (EDX) spectroscopy
Collection and identification of plant materials
The leaves, stems and seeds of Foeniculum vulgare were accumulated from the shrubbery where it develops in wealth in Alice, Eastern Cape Province of South Africa. The plant was identified by Tony Dold of Rhodes University, with voucher specimen number ASO 2014/2. It was later kept in the Giffen Herbarium of the University of Fort Hare, Alice.
Scanning electron microscopy (SEM)
Crisp leaves, seeds and stems (transverse) were cut into fragments of 4–6mm long and settled in 2.5% glutaldehyde with pH 7.3 for 24 hours. Specimens were washed for 15-30 minutes with the 0.1M phosphate buffer. Each specimen was later flushed in refined water and got dried out in an evaluated arrangement of ethanol 10–100% for 20 min for every wash. The areas were dried in a Hitachi HCP-2 basic point dryer and mounted on aluminium stubs with two fold sided carbon covered sputter covering with gold-palladium (Elko IB-3 Ion Coater). The samples were inspected at different magnifications utilizing JEOL (JSM-6390LV) scanning electron magnifying instrument (SEM) that was worked at 10– 15 kV quickened voltage. The vitality dispersive X-beam spectroscopy (EDX) included both settling and drying out method as in SEM, while the basic examination was finished utilizing vitality dispersive X-beam analyser which was coupled to SEM, made by Thermo Electron Corporation, 6733B-IUUSN, USA. The Noran framework six programming was utilized for imaging. The structures observed in the leaf, stem and seed were: stomata types, an outline of epidermal cells and type of crystals present.
The appearance of the epidermal cells and stomata distribution
The leaves and stems of Foeniculum vulgare that were observed under SEM showed a considerable amount of amphistomatous densely distributed in the leaf and stem surfaces (Figure 1) (Figure 2) on the epidermal surfaces. Large portions of the stomata saw on the adaxial surface were completely opened while the few located on the abaxial surface were much shut. The densely amphistomatous in F. vulgare suggest its habitat which is in the open with an adequate amount of water intake because plants growing in shady and drought-prone environments have sparsely distributed stomata.3 The stomata are diacytic while the guard cells are elliptical in shape; this is in concurrence with the report of Annal.4 On the taxonomic significance of leaf characters in some species of Apiaceae. The stomata are microscopic openings on the surfaces of all plants which primarily function for the exchange of gas, during respiration and photosynthesis. From the report of Okubamichael.5 it was noted that during the opening of stomata the rate of transpiration increases which also affects the increase in absorption of water. In Foeniculum vulgare, the adaxial and abaxial stomata were observed to be opened which implies that the rate of transpiration is high in this plant. It has also been ascertained that stomata in a plant are mostly closed at night which means that the rate of transpiration will be reduced at night. It was further observed in the stems (Figure 2) that there is the presence of a longitudinal form of lenticels in the stem, because lenticels are commonly found in the stems (a form of pore) of most plants which fills in as a site of gas trade in the plant when the stomata close during the evening. Lenticels are additionally, shockingly, a site where pathogens, for example, parasites, microbes and infections can enter the plant. This to the best of our insight is the main report on foliar lenticels and ultra morphological studies of Foeniculum vulgare. This we believe could provide more information on the structure of F.vulgare (Figure 3).
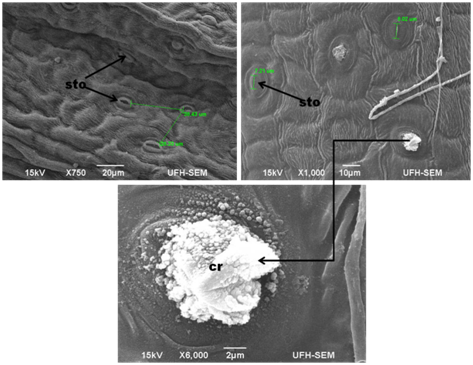
Figure 1 SEM photograph of a- epidermal surfaces and stomata distribution (x750) showing the distance between stomata b- crystal deposit from a stoma (x 1000) c- shows styloid crystals at higher magnification x 6000 in Foeniculum vulgare.
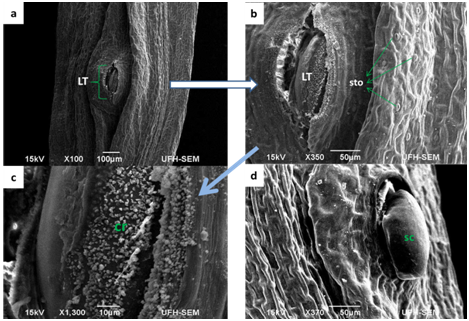
Figure 2 Scanning Electron Micrograph of Foeniculum vulgare stem (a & b) transverse sectioning of the stem showing the lenticels (LT); (c & d) stem having mineral sediments.
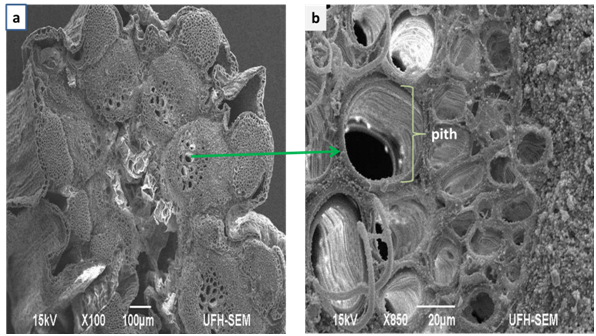
Figure 3 Scanning Electron Micrograph of Foeniculum vulgare stem (a) cross sections showing the inner rings of xylem tissues; (b) showing the pith.
Figure 3 demonstrates a cross-segment of the vascular heap of the xylem (orchestrated in ringed shapes) and phloem tissues of the stem. The xylem tissue fills in as a vessel for transporting water, broke up minerals and strands which bolster the plant.6 The phloem transports natural substances through the stem. It additionally demonstrates the nearness of beads of mineral dregs on the phloem tissue which is translocated to all parts of the plant. It also shows the structure of pith which help to store water and starch and also help in exchange of gases through the intercellular air spaces. These features indicate that Foeniculum vulgare contain a lot of mineral deposit and can be of significance to microorganisms and man.
The appearance of crystals and elemental composition
In this study, styloid crystals were observed in Foeniculum vulgare (Figure 1) (Figure 2c). Plants collect precious stones of calcium oxalate in an assortment of shapes, for example, dust, sand, kaleidoscopic, raphides or styloid sizes, sums, and spatial areas.7,8 Crystals are also believed to play roles in the exchange of cellular ion balance and tissue rigidity and support in detoxification of harmful metals.9 In spite of the fact that, the significance of crystals and their make-up is generally obscure, despite the fact that different capacities have been ascribed to their essence.10 (Figure 4) The X-ray examination of the leaf, stem and seed in F.vulgare produced spectra of the accompanying micro and macro mineral components; Calcium (Ca), Oxygen (O), Sodium (Na), Aluminium (Al), Silicon (Si), Phosphorus (P), Sulphur (S), Chlorine (Cl), Potassium (K), Iron (Fe), Bromine (Br), Carbon (C), Magnesium (Mg) (Figures 4, 5 & 6). The major elemental constituents of Foeniculum vulgare are Ca, Na and S while Al, K and Mg which were present in minute quantity (Table 1). Calcium is fundamental for muscle withdrawal, ocytes initiation, building solid bones and teeth blood thickening, nerve drive controlling pulse and liquid adjust inside cells3 which could be correlated to the presence of crystals in this plant. The high percentage of calcium observed in this plant may be required for free movements of nutrients within the plant tissue. Calcium is also assumed to initiate closure of the stomata and hence plays a role in transduction. Calcium was recorded on the abaxial surface and little quantity in the crystal of F.vulgare (Table 1); hence, this could suggest why the stomata observed on the abaxial surface in the SEM photomicrographs were tightly closed while the adaxial surface without Ca deposits had most of its stomata fully opened. Sulphur is involved in cellular respiration, protein synthesis, cell regeneration and blood cleansing which helps the cells to use oxygen efficiently. Magnesium has been accounted for, to be useful in battling coronary illness, stroke and in cell repair in people. The body needs a little measure of sodium to help keep up circulatory strain and ordinary capacity of muscle and nerves.3 The elements and morphological features observed in this plant may be responsible for the therapeutic actions of Foeniculum vulgare. A further study to evaluate the metabolites present in this plant is in progress (Figure 5) (Figure 6).
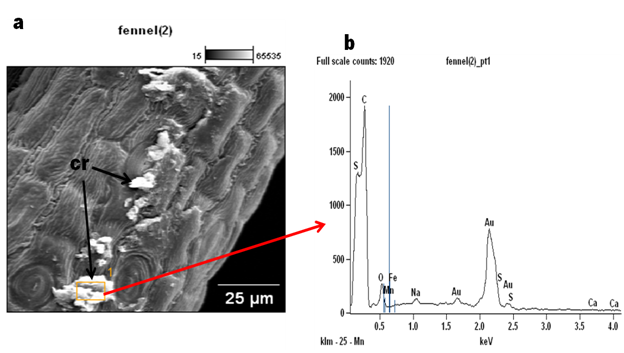
Figure 4 Scanning Electron Micrograph of Foeniculum vulgare stem (a) cross sections showing the inner rings of xylem tissues; (b) showing the pith.
Elemental composition |
Leaf (%) |
Stem (%) |
Seed (%) |
Calcium (Ca) |
3.96 |
5.54 |
2.3 |
Oxygen (O) |
41.6 |
28.09 |
37.84 |
Sodium (Na) |
0.47 |
0.42 |
0.16 |
Aluminium ( Al) |
- |
- |
- |
Silicon (Si) |
- |
- |
2.36 |
Phosphorus (P) |
- |
- |
- |
Sulphur (S) |
0.7 |
4.58 |
2.07 |
Chlorine (Cl) |
- |
- |
- |
Potassium (K) |
0.72 |
0.83 |
- |
Iron (Fe) |
- |
0.28 |
0.44 |
Bromine (Br) |
- |
- |
1.27 |
Carbon (C) |
32.09 |
16.19 |
39.86 |
Magnesium (Mg) |
0.76 |
0.54 |
0.24 |
Table 1 Elemental composition (%) of Foeniculum vulgare leaves as shown by Energy Dispersive X-ray (EDX) analysis
The epidermal surfaces of leaves and stems of Foeniculum vulgare were investigated using Scanning Electron Microscopy (SEM) equipped with Energy Dispersive X-ray (EDX) spectroscopy. The stoma is well distributed over the surfaces of both leaves and stems with no evidence of a trichome. Styloid crystals were observed on the leaves surfaces. The major elemental constituents of Foeniculum vulgare were Ca, Na and S while Al, K and Mg were also present in minute quantity. The absence of trichomes which is a major storage and secretory structure (for major phytochemicals) of leaves suggests that other specialized structures such as crystals may be responsible for their storage of the phytochemicals.
This investigation was bolstered by the Govan Mbeki Research and Development Center, University of Fort Hare and Medicinal Plants and Economic Development (MPED) Research Center. The authors say thanks to MS Famewo EB for specialized help.
We pronounced that we have no budgetary or individual connections which may have improperly affected us in composing this paper.
The authors declare that they have no competing interest.

©2019 Ayodele, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.