Advances in
eISSN: 2373-6402


Research Article Volume 9 Issue 1
1Department of Crop Science, Faculty of Agriculture, Damanhour University, Egypt
2Department of Horticulture, Faculty of Agriculture, Damanhour University, Egypt
Correspondence: Hanaa M Abouzied, Department of Crop Science, Faculty of Agriculture, Damanhour University, Damanhour, Egypt
Received: January 18, 2019 | Published: February 22, 2019
Citation: Abouzied HM, Farag KM. Effect of foliar application of natural compound Lysophosphatidylethanolamine (LPE), potassium and magnesium on yield attributes of soybean (Glycine max (L)) in non- arid. Adv Plants Agric Res. 2019;9(1):300-309. DOI: 10.15406/apar.2019.09.00439
The objectives of this work were to evaluate the effect of the natural compound Lysophosphatidylethanolamine (LPE), and the essential elements K and Mg in sulphate form foliar spray on yield performance of soybean (Glycine max (L.) Merrill) used either singly or in combinations. The field trials were conducted at two locations, Abu Hummus (non-arid condition) and El-Abadya (arid condition), El-Beharia Governorate during the growing season 2017using two soybean cultivars Giza21 and Giza111. Nine treatments that included control, Lisophos at 50ppm, Lisophos at 100ppm, Lisophos (50ppm) plusKSO4 1% (w/v), Lisophos (100ppm)plus KSO4 1% (w/v), KSO4 1% (w/v), MgSO4 1% (w/v), Lisophos (50ppm)plus Mg SO4 1% (w/v) and Lisophos (100ppm)plus MgSO4 1% (w/v)were applied during the stages R1 (bloom beginning) and R2 (full bloom). Pod fresh weight/plant (g), pod number/plant, 1000 seed weight (g), number of seed/10 pods, number of main branches/plant, length of main branch (cm), stem dry weight/plant (g), dry weight of seed/10 pods (g) and fresh weight/10 pods(g) were evaluated. At the location of Abu Hummus where represented an old Delta soil, vigorous growth was observed in almost all traits and the opposite was shown in El-Abadya location, which represented the newly reclaimed soil. In general, the performance of Giza111 was greater than Giza21 in most of the studied traits across the two locations. Under the arid location (El-Abadya) foliar spray with Lisophos 100ppm + MgSO4 1% (w/v) enhanced pod fresh weight/plant, pod number /plant, stem dry weight and number of main branches/plant. In the non-arid location (Abu Hummus), pod number /plant was highly promoted by Lisophos 100ppm, while high fresh weight for pod/plant was increased by the application of MgSO4 1% (w/v).In addition Lisophos 100ppm + MgSO4 1% (w/v) resulted in an increased branching in both of the studied cultivars. In conclusion, this study provided evidence that the foliar spray treatments could enhance the yield attributes more than control in soybean especially during the reproductive stages.
Soybean (Glycine max (L.) Merrill) is one of the legumes that belong to the Papilionaceae. Soybean is an important oil crop, providing millions of people with oil, protein and other chemical components. Soybean grass is self-pollinated plant, rich in vegetative growth and branching, which branches a lot from the bottom if the distances of planting are wide; It can grow up to two meters, depending on the variety and the environmental conditions. In Egypt It began to be cultivated at commercial scale in the early of 70s, currently Agricultural Research Center (Egypt) distributed four newly certified soybean seed varieties i.e Giza 21, Giza 22, Giza 25, and Giza 111.Soybeans crop are cultivated in Middle and Upper Egypt (southern Egypt).Plant growth regulators are known to have different roles in crops performance and developments during their different growth stages, they are known to delay leaf senescence, enhance effective partitioning of photo synthetic accumulates from source to sink, affect flower formation, fruit set and seed development and yield.1 Soybean plants produce a lot of floral buds, but most of them fail to grow pods and abort during development.2 Lyso-phosphatidyl-ethanol-amine (LPE) (commercially available as Lisophos) is used commercially as a plant bio-regulator to improve plant productivity and quality. It is found naturally in plant, animals and human membrane. When exogenously applied to plant it can enhance fruit ripening and coloration while delaying leaf senescence. It can also mitigate the damage of different abiotic stress, it extends the vase life of cut flowers, moreover it increases fruit set and increases seed germination and yield.3–6 The present study involved two soybean varieties viz. Giza21 and Giza 111 were chosen to study the influence of Lysophosphatidylethanolamine (LPE) on yield components. Plant growth can be maximized by foliar spraying with plant growth regulators or essential elements.7 Foliar application can quickly relief plants from adverse environmental condition such as stress due nutrients deficiency, water scarcity, high temperature, pests and diseases.7 Foliar nutrient application can be a worthy approach to rise crop yield, and produce fast response in a short time.8,9 Scientists found that foliar spray encouraging nutrition balance inside the plant and over a field.10–12 However, inadequate research has examined the combination between macronutrient foliar fertilizers and plant growth regulators specially Lysophosphatidylethanolamine (LPE) as well as in annual crops. Plant growth regulators (PGRs) have been used for years on horticulture crops, only in the last few decades it has become more common to use them as a means to improve yield and management of seed crops. Currently Little is well-known about the roles of plant growth regulators in expression yield components, yield and seed qualities of soybean.13 The objectives of this study aimed to study the effect of Lysophosphatidylethanolamine (LPE) and essential elements such as potassium and magnesium in maximizing soybean yield attributes through foliar application independently or combined.
Two Field experiments were carried out at two locations, Abu Hummus represented non-arid location and El-Abadya an arid newly reclaimed location El-Beharia Governorate, Egypt during the growing season 2017 to evaluate yield attributes of two soybean varieties for maximizing seed yield using 8 foliar spray treatments including Lysophosphatidylethanolamine (LPE), MgSO4, KSO4, Lysophosphatidylethanolamine (LPE) combined with MgSO4 orKSO4 (Table 1). Two soybean varieties (Giza21 and Giza111) were used Table 2 shows their common names, pedigree, origin, maturity group, growth habit and growth habit of the parental soybean varieties. The varieties Giza 21 and Giza 111 are characterized for improved quality, and are insect-resistance and need low amount of nitrogenous fertilizer.14 The seed of cultivars was obtained from Food Legumes Research Section, Agricultural Research Center Giza, Egypt. Spraying of chemical treatments was done two times at during the R1 (Beginning of flower opening) and R2 (Full bloom about 50% flowering. The two field experiments were sown on 11th and 12th of May during 2017season for Abu Hummus and El Abadya respectively. Soybean seeds were planted in hill spaced 20cm on the two sides of the ridge. Each hill received 4 seeds and was thinned to three plants per hill 21 days after sowing. A split plot distribution with three replications was used. Soybean varieties were randomly assigned to the main plots and chemical treatments allocated in sub-plots. The area of sub-plot was 10.8m2 with each plot consisting of six ridges and each ridge was 3.0min length and 0.6m in width. Normal recommended standard cultural practices for growing soybean crop were used. The studied traits at harvest, were pod fresh weight/plant(g), pod number/plant, 1000 seed weight(g), number of seed/10 pods, number of main branches/plant, length of main branch(cm), stem dry weight/plant(g), dry weight of seed/10 pods(g), fresh weight/10 pods(g) . Data were collected at final harvesting of the crop when the foliage turned pale yellow.
|
Treatment |
Concentrations |
|
T1 |
Control |
|
T2 |
Lisophos 50ppm |
|
T3 |
Lisophos 100ppm |
|
T4 |
Lisophos 50ppm+KSO4 1% (w/v) |
|
T5 |
Lisophos 100ppm + KSO4 1% (w/v) |
|
T6 |
KSO4 1% (w/v) |
|
T7 |
MgSO4 1% (w/v) |
|
T8 |
Lisophos 50ppm + Mg SO4 1% (w/v) |
|
T9 |
Lisophos 100ppm + MgSO4 1% (w/v) |
Table 1 The applied foliar spray treatments and their concentrations
|
Varieties |
Pedigree |
Origin |
Maturity group |
Growth habit |
|
Giza 111 |
Crawford x Celeste |
Egypt |
IV |
Indeterminate |
|
(late ) |
||||
|
Giza 21 |
Crawford x Celeste (late) |
Egypt |
IV |
Indeterminate |
Table 2 The common names, pedigree, origin, maturity group, growth habit and maturing dates of the parental soybean varieties
The effects of treatments on soybean variables were assessed by two–way analysis of variance for split–plot design Soybean varieties were randomly assigned to the main plots and chemical treatments allocated in sub-plots, with three replications. All data were assessed by analysis of variance (ANOVA), performed using SAS (SAS Institute, 1988). Since significant interactions existed for most independent variables in this study, each location was analyzed separately.15 Mean comparisons were done using Least Significant Differences (LSDs) method at 5% level of probability to compare differences between the means.16 Figures were created using Microsoft Excel.
Environmental condition is important for the progress of soybean plant architecture that promotes grain yield production. At the location of Abu Hummous, vigorous growth was observed in almost all traits while less plant vigor was shown under arid condition El-Abadya location, (Table 3) (Table 4) (Figure 1). At the studied parameters, exogenous chemical treatments application significantly altered pod fresh weight/plant, pod number/plant, stem dry weight /plant, dry weight of seed/10pods and fresh weight of 10 pods at the two locations (Table 4 & Figure 1).1000 seed weight (g), Number of main branch/plant and length of main branch, were significant different at non arid location only. Number of seed/10 pod was insignificantly affected by each of chemical treatments, cultivars, and interaction among them (Table 4). Significant differences among genotypes for characters viz, pod fresh weight/plant, pod number /plant were found across the two locations, while stem dry weight and dry weight of seed/10pods(g) were significantly different on Elabaya only, on the other hand varietal variations were significantly affected number of main branches/plant and Fresh weight pod/10 pods(g) at Abu hummus only while the other traits were statistically non-significant at both locations (Table 4). Genotypes x treatments interaction were significantly different in almost all the studied traits either across the two locations or at one location and were in significantly different for number of seed/10 pods, fresh weight of pod/ 10pods (g) and length of main branch across the two locations (Table 4). Pod fresh weight/plant Soybean pod fresh weight/plant was affected by both the chemical treatments and cultivars, with an interaction occurring between these factors at the two locations (Table 4, Figure 1). In general, Giza111 produced higher pod fresh weight/plant than Giza21 (Table 5).
|
Pod fresh weight/plant |
|
|
|
|
|
|
|
|
|
||
|
Treatments |
|||||||||||
|
L |
Cultivars |
C |
Lisophos 50ppm |
Lisophos 100ppm |
Lisophos 50 ppm+KSO4 1% (w/v) |
Lisophos 100ppm + KSO4 1% (w/v) |
KSO4 1% (w/v) |
MgSO4 1% (w/v) |
Lisophos 50ppm + Mg SO4 1% (w/v) |
Lisophos 100ppm + MgSO4 1% (w/v) |
mean |
|
L1 |
Giza 21 |
158.9 |
149.4 |
219.4 |
183.9 |
145.6 |
181.6 |
190.7 |
255.3 |
173.2 |
184b |
|
Giza111 |
169.3 |
260.6 |
259.5 |
223.3 |
195.5 |
191.3 |
328 |
216.7 |
176.6 |
224.5a |
|
|
Mean |
164.1f |
205.0c |
239.4b |
203.6c |
170.6e |
186.4d |
259.4a |
236.0b |
174.9e |
||
|
Giza 21 |
62.8 |
60 |
76.5 |
60.6 |
41.5 |
45 |
70 |
55.7 |
96.3 |
63.1a |
|
|
L2 |
Giza111 |
55.3 |
112.3 |
97 |
96.5 |
109.7 |
109.3 |
109.8 |
104.5 |
99.9 |
99.3b |
|
Mean |
59.0g |
86.15c |
86.7c |
78.5de |
75.6f |
77.1ef |
89.9b |
80.1d |
98.15a |
||
|
Pod number /plant |
|
|
|
|
|
|
|
|
|
|
|
|
Treatments (T) |
|||||||||||
|
L |
Cultivars |
C |
Lisophos 50ppm |
Lisophos 100ppm |
Lisophos 50 ppm+KSO4 1% (w/v) |
Lisophos 100ppm + KSO4 1% (w/v) |
KSO4 1% (w/v) |
MgSO4 1% (w/v) |
Lisophos 50ppm + Mg SO4 1% (w/v) |
Lisophos 100ppm + MgSO4 1% (w/v) |
mean |
|
L1 |
Giza 21 |
216.9 |
291.1 |
331.1 |
280.2 |
212.6 |
292.4 |
307.9 |
420.4 |
235 |
287.5b |
|
Giza111 |
259.2 |
346.1 |
547.5 |
411 |
386.6 |
254.1 |
377.6 |
355.6 |
248.2 |
354.0a |
|
|
Mean |
238.05f |
318.6d |
439.3a |
345.6c |
299.6d |
273.2e |
342.7c |
388.0b |
241.6f |
||
|
L2 |
Giza 21 |
132 |
95.1 |
134.3 |
109 |
123.3 |
114.4 |
150.4 |
113.5 |
196 |
129.8b |
|
Giza111 |
120.8 |
202.4 |
187 |
158.9 |
243.9 |
252.9 |
210.1 |
210.9 |
249.8 |
204.0a |
|
|
Mean |
126.4g |
148.7e |
160.6d |
133.9f |
183.6b |
183.6b |
180.2c |
162.2d |
222.9a |
||
|
1000 seed weight (g) |
|
|
|
|
|
|
|
|
|
||
|
Treatments |
|||||||||||
|
L |
Cultivars |
C |
Lisophos 50ppm |
Lisophos 100ppm |
Lisophos 50 ppm+KSO4 1% (w/v) |
Lisophos 100ppm + KSO4 1% (w/v) |
KSO4 1% (w/v) |
MgSO4 1% (w/v) |
Lisophos 50 ppm + Mg SO4 1% (w/v) |
Lisophos 100ppm + MgSO4 1% (w/v) |
mean |
|
L1 |
Giza 21 |
334.6 |
273.4 |
237.7 |
198.2 |
206.7 |
199.6 |
194.3 |
215.9 |
189.4 |
227.7a |
|
Giza111 |
319.3 |
340.7 |
289 |
208.7 |
380.6 |
240.5 |
234.8 |
207.7 |
195.6 |
268.5a |
|
|
Mean |
326.9a |
307.1a |
263.4a |
203.5a |
293.6a |
220.0a |
214.6a |
211.8a |
192.5a |
||
|
L2 |
Giza 21 |
144.8 |
129.7 |
132.1 |
150.7 |
130.3 |
117.3 |
111.6 |
112.8 |
143.7 |
130.3a |
|
Giza111 |
100.4 |
116.63 |
107.3 |
125.1 |
141.6 |
149.8 |
118.4 |
139.9 |
106.2 |
122.8a |
|
|
Mean |
122.6a |
123.1a |
119.7a |
137.9a |
135.9a |
133.6a |
115.0a |
126.3a |
124.95a |
||
|
Number of seed /10 pod |
|
|
|
|
|
|
|
|
|
||
|
Treatments |
|||||||||||
|
L |
Cultivars |
C |
Lisophos 50ppm |
Lisophos 100ppm |
Lisophos 50 ppm+KSO4 1% (w/v) |
Lisophos 100ppm + KSO4 1% (w/v) |
KSO4 1% (w/v) |
MgSO4 1% (w/v) |
Lisophos 50ppm + Mg SO4 1% (w/v) |
Lisophos 100ppm + MgSO4 1% (w/v) |
Mean |
|
L1 |
Giza21 |
26 |
24.3 |
27.3 |
27.6 |
26.6 |
27.3 |
29 |
25.6 |
26 |
26.6a |
|
Giza111 |
24.3 |
27.3 |
25 |
27.3 |
26 |
24.6 |
28.3 |
25.6 |
28.6 |
26.3a |
|
|
Mean |
25.1a |
25.8a |
26.1a |
27.5a |
26.3a |
26a |
28.6a |
25.6a |
27.3a |
||
|
L2 |
Giza21 |
22.6 |
24 |
23.3 |
24 |
26.6 |
26.6 |
27 |
24 |
27.3 |
27a |
|
Giza111 |
26 |
28 |
24 |
24.6 |
26.3 |
28 |
25.6 |
28 |
28 |
27a |
|
|
Mean |
24.3a |
26a |
23.6a |
24.3a |
25.1a |
27.3a |
26.1a |
27.5a |
25.6a |
||
|
Number of main branch/plant |
|
|
|
|
|
|
|
|
|
||
|
Treatments |
|||||||||||
|
L |
Cultivars |
C |
Lisophos 50ppm |
Lisophos 100ppm |
Lisophos 50 ppm+KSO4 1% (w/v) |
Lisophos 100ppm + KSO4 1% (w/v) |
KSO4 1% (w/v) |
MgSO4 1% (w/v) |
Lisophos 50ppm + Mg SO4 1% (w/v) |
Lisophos 100ppm + MgSO4 1% (w/v) |
Mean |
|
L1 |
Giza 21 |
5.1 |
4.2 |
5.7 |
5.4 |
5.3 |
5.1 |
5.6 |
6.7 |
7.8 |
5.6b |
|
Giza111 |
6.6 |
7.6 |
10 |
8.9 |
7.2 |
8 |
11.2 |
7.3 |
10.9 |
8.6a |
|
|
Mean |
5.8d |
5.95d |
7.8bc |
7.1cd |
6.2d |
6.5d |
8.4ab |
7.0cd |
9.3a |
||
|
L2 |
Giza 21 |
5.2 |
4.4 |
4.6 |
5.6 |
4.6 |
3.9 |
4.6 |
5.5 |
6 |
4.9a |
|
Giza111 |
6.6 |
8.4 |
5.6 |
5.2 |
6.4 |
7.3 |
6.2 |
6.6 |
8.1 |
6.7a |
|
|
|
Mean |
5.95a |
6.45a |
5.1a |
5.4a |
5.5a |
5.6a |
5.6a |
5.4a |
6.1a |
|
Table 3 Soybean yield parameters as affected by foliar treatments and cultivars at two locations in El-Beharia governorate
|
Length of main branch |
|
|
|
|
|
|
|
|
|
||
|
Treatments |
|||||||||||
|
L |
Cultivars |
C |
Lisophos 50ppm |
Lisophos 100ppm |
Lisophos 50 ppm+KSO4 1% (w/v) |
Lisophos 100ppm + KSO4 1% (w/v) |
KSO4 1% (w/v) |
MgSO4 1% (w/v) |
Lisophos 50ppm + Mg SO4 1% (w/v) |
Lisophos 100ppm + MgSO4 1% (w/v) |
mean |
|
L1 |
Giza 21 |
89.6 |
109 |
116 |
124 |
117.3 |
127 |
139 |
129.3 |
129.6 |
120a |
|
Giza111 |
80.3 |
103.6 |
107.3 |
114.3 |
110.3 |
101.6 |
119.6 |
127.3 |
109 |
108a |
|
|
mean |
85b |
106.3a |
111.6a |
119.1a |
114.3a |
113.8a |
114.3a |
128.3a |
129.3a |
||
|
Giza 21 |
110.3 |
110 |
113 |
110.3 |
92 |
95 |
106.6 |
100.3 |
111.1 |
105a |
|
|
L2 |
Giza111 |
83.7 |
85.7 |
112.7 |
115.3 |
112.7 |
90 |
97.3 |
193 |
101 |
100a |
|
mean |
97a |
97.8a |
112.8a |
112.8a |
101.8a |
92.5a |
102a |
101.6a |
106.3a |
||
|
Stem Dry weight |
|
|
|
|
|
|
|
|
|
|
|
|
Treatments |
|||||||||||
|
L |
Cultivars |
C |
Lisophos 50ppm |
Lisophos 100ppm |
Lisophos 50 ppm+KSO4 1% (w/v) |
Lisophos 100ppm + KSO4 1% (w/v) |
KSO4 1% (w/v) |
MgSO4 1% (w/v) |
Lisophos 50ppm + Mg SO4 1% (w/v) |
Lisophos 100ppm + MgSO4 1% (w/v) |
mean |
|
L1 |
Giza 21 |
9.7 |
20.7 |
23.8 |
22 |
18.8 |
26 |
26.2 |
20.6 |
13 |
|
|
Giza111 |
17.5 |
25.7 |
32 |
23.6 |
24.1 |
17.6 |
17.3 |
18.8 |
14.6 |
21.2a |
|
|
mean |
13.6f |
23.2b |
27.9a |
22.8bc |
21.4d |
21.8cd |
21.7cd |
19.7e |
13.8f |
20.1a |
|
|
L2 |
Giza 21 |
7.3 |
8.6 |
10.2 |
9.4 |
8.5 |
7.2 |
8.7 |
7.2 |
10.8 |
8.6a |
|
Giza111 |
7 |
9.2 |
11.4 |
12.4 |
9.2 |
13.8 |
11.8 |
7.9 |
11 |
10.4a |
|
|
mean |
7.1b |
8.9ab |
10.8a |
10.9a |
8.9ab |
10.5a |
10.3a |
7.5b |
10.9a |
||
|
Dry weight of seed /10 pods (g) |
|
|
|
|
|
|
|
|
|||
|
Treatments |
|||||||||||
|
L |
Cultivars |
C |
Lisophos 50ppm |
Lisophos 100ppm |
Lisophos 50 ppm+KSO4 1% (w/v) |
Lisophos 100ppm + KSO4 1% (w/v) |
KSO4 1% (w/v) |
MgSO4 1% (w/v) |
Lisophos 50ppm + Mg SO4 1% (w/v) |
Lisophos 100ppm + MgSO4 1% (w/v) |
mean |
|
L1 |
Giza 21 |
8.3 |
25.1 |
18.7 |
18.3 |
9 |
22.3 |
7 |
12.1 |
10 |
14.5a |
|
Giza111 |
6.4 |
7.2 |
15.5 |
10.7 |
6 |
15.7 |
14.6 |
23.5 |
19.3 |
14.5a |
|
|
mean |
7.3c |
16.2ab |
17.1ab |
14.5b |
7.5c |
19.0a |
10.8c |
17.8ab |
14.7b |
||
|
L2 |
Giza 21 |
5.6 |
6.5 |
8.1 |
16.9 |
24.7 |
17.9 |
24.2 |
13.4 |
20.2 |
13.2b |
|
Giza111 |
6.4 |
8.2 |
12.6 |
11.4 |
18.7 |
14.8 |
16.5 |
13.1 |
16.9 |
15.3a |
|
|
mean |
6f |
7.3f |
10.3e |
14.1d |
21.7a |
16.4c |
20.35a |
13.2d |
18.5b |
||
|
Fresh weight / 10 pods (g) |
|
|
|
|
|
|
|
|
|
||
|
Treatments |
|||||||||||
|
L |
Cultivars |
C |
Lisophos 50ppm |
Lisophos 100ppm |
Lisophos 50 ppm+KSO4 1% (w/v) |
Lisophos 100ppm + KSO4 1% (w/v) |
KSO4 1% (w/v) |
MgSO4 1% (w/v) |
Lisophos 50ppm + Mg SO4 1% (w/v) |
Lisophos 100ppm + MgSO4 1% (w/v) |
mean |
|
L1 |
Giza21 |
7.4 |
11.7 |
9.1 |
8.3 |
8 |
8.1 |
10.3 |
7.6 |
9.7 |
8.9b |
|
Giza111 |
10.3 |
14 |
12.8 |
10.4 |
11.5 |
10.5 |
11.7 |
11.1 |
11.2 |
11.5a |
|
|
mean |
8.8c |
12.8a |
10.9b |
9.4bc |
9.8bc |
9.3bc |
11.0b |
9.3bc |
10.5bc |
||
|
L2 |
Giza21 |
4 |
5.7 |
5.4 |
5.5 |
5.4 |
5.2 |
5.5 |
5.5 |
5 |
5.2a |
|
Giza111 |
4.2 |
4.8 |
4.5 |
4.8 |
5.5 |
5.5 |
4.7 |
4.9 |
4.4 |
4.8a |
|
|
|
mean |
4.0b |
5.25ab |
4.9a |
5.1a |
5.4a |
5.3a |
5.1a |
5.4a |
4.6a |
|
Table 4 Soybean yield parameters as affected by foliar treatments and cultivars at two locations in El-Beharia governorate
|
|
Source of variance |
Df |
Mean square |
|
Traits |
|
|
AbuHummus |
|
pod fresh weight/plant |
Genotypes |
1 |
21933.27** |
|
Treatments |
8 |
6906.23** |
|
|
Genotypes*treatments |
8 |
4490.43** |
|
|
pod number/plant |
Genotypes |
1 |
59660.48** |
|
Treatments |
8 |
26621.69** |
|
|
Genotypes*treatments |
8 |
13119.48** |
|
|
1000 seed weight (g) |
Genotypes |
1 |
22464.48ns |
|
Treatments |
8 |
15236.50* |
|
|
Genotypes*treatments |
8 |
4911.41ns |
|
|
Number of seed /10 pod |
Genotypes |
1 |
1.185182ns |
|
Treatments |
8 |
7.26ns |
|
|
Genotypes*treatments |
8 |
5.93ns |
|
|
Number of main branch/plant |
Genotypes |
1 |
118.81** |
|
Treatments |
8 |
8.421** |
|
|
Genotypes*treatments |
8 |
3.315** |
|
|
Length of main branch |
Genotypes |
1 |
1920.07ns |
|
Treatments |
8 |
1050.64** |
|
|
Genotypes*treatments |
8 |
93.69ns |
|
|
Stem Dry Weight |
Genotypes |
1 |
18.02ns |
|
Treatments |
8 |
123.78** |
|
|
Genotypes*treatments |
8 |
61.26** |
|
|
Dry weight of seed /10 pods (g) |
Genotypes |
1 |
23.20ns |
|
Treatments |
8 |
113.53** |
|
|
Genotypes*treatments |
8 |
132.53** |
|
|
Fresh weight of pod/ 10 pods (g) |
Genotypes |
1 |
89.44** |
|
Treatments |
8 |
9.46** |
|
|
|
Genotypes*treatments |
8 |
1.06ns |
Table 5 Analysis of variance for studied traits of Soybean cultivars across the two locations Abu Hummus (non- arid) and El Abadia (arid)
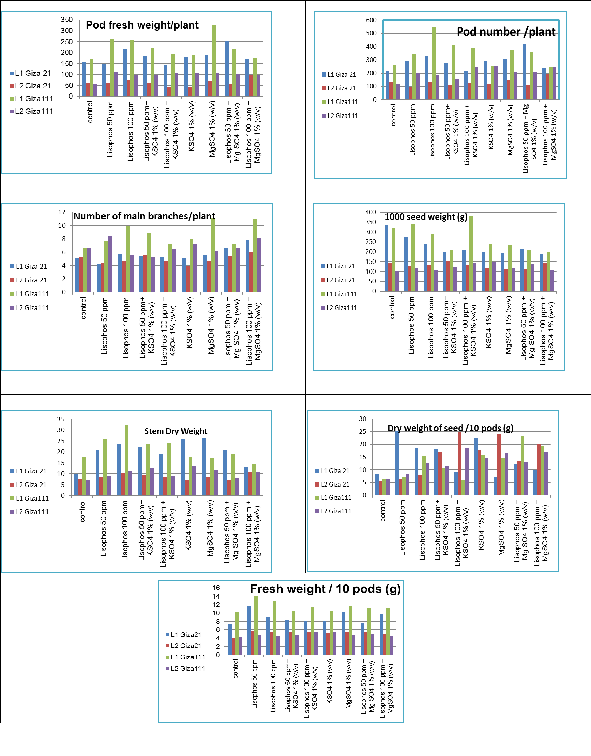
Figure 1 Soybean yield parameters as affected by foliar treatments and cultivars at two locations in El-Beharia governorate. (L1= Abu humous, L2= El Abadya), vertical axis represented mean value of the studied traits.
*,**Significant at 5% and 1% probability levels, respectively, ns=non significant.
Increased in pod fresh weight/plant due to chemical application was observed at each of the two studied locations (Table 5), Treatments effects on pod fresh weight/plant were inconsistent among locations. In Abu Hummus MgSO4 1% (w/v) gave the highest weight. On the other hand in El-Abadia, Lisophos 100ppm + MgSO4 1% (w/v) gave the highest weight. Varietal differences in response to the applied treatments were shown as example at the non arid location Lisophos 50ppm + Mg SO4 1% (w/v) gave the best response with Giza21, while MgSO4 1% (w/v) showed the best response with Giza111 (Table 5). Encouraging response for yield was shown with different crops sugar beet (Beta vulgaris L.),17 soybean (Glycine max),18 and fava bean (Viciafaba)19 when treated exogenously with Mg.20 reported that Mg rates of 540 and 890 g ha-1 increased in 325 and 737kg ha-1 the yield of soybean and corn, respectively, regardless of the phenological growth stages. Teklić et al.,21 found that soybean cultivars vary in their response to Mg applied as a foliar spray. Cowan22 highlighted the role Lisophos in the treated plant in delaying senescence in leaf and fruits, enhanced fruit quality and decreased susceptibility to abiotic and biotic stresses.
Pod number /plant: This trait significantly varied between cultivars at the two locations (Table 4). Across the two studied locations Giza111 had significantly higher pod number/plant than the other cultivar. While differences in pod number/plant due to the application were also observed at the two locations (Table 4, Figure 1). A significant chemical treatment x cultivar interaction was found across the two locations. In Abu Hummus location Lisophos 100ppm was the best while at the newly reclaimed area (El-abadia) Lisophos 100ppm + MgSO4 1% (w/v) had the higher trait value. Giza111 showed superiority in pod number/plant with Lisophos 100ppm at Abu Hummus while Giza 21 showed highest potential of pod number/plant when received Lisophos 50ppm + Mg SO4 1% (w/v) treatment. Giza111 significantly performed high with KSO4 1% (w/v) in the arid environment, while Giza21 was best with Lisophos 100ppm + MgSO4 1% (w/v).Various factors, such as environmental condition, concentration of the used plant growth regulator, varietal genetic makeup can interfere the performance of soybean plants. Number of pods and the development of pods is determined by the assimilate providences and the balance of endogenous plant growth regulators. Exogenous application and time of application of plant growth regulators influences yield components of soybean.23 Several studies reported that exogenous application of plant growth regulators improve yield pod set of soybean.24–26 Thousand seed weight (g): Treatments were significantly affected the traits in the Abu Hummus location and were not significant at the El- Abadya.
In addition interaction between cultivars x treatments was found to be significant in the newly reclaimed area only. In Abu Hummus the control gave the highest weight with Giza21 while the Lisophos 100ppm + KSO4 1% (w/v) gave the highest value with Giza111. Furthermore Lisophos 50ppm+KSO4 1% (w/v) was found to be better with Giza21 at the newly reclaimed area (El-Abadya). In additionKSO4 1% (w/v) enhanced Giza111 in El-Abadya location. Seed weight is unlike other yield components such as pod number per plant which is highly affected by the environment, and number of seeds per pod which is highly genotype dependent, the dominant factors affecting seed weight are not yet well established.27 Previous studies demonstrated that seed weight of soybean is highly heritable,28 on other hand the environmental conditions have great influence upon this traits in fluctuating its size. Several studies reported positive effect of exogenous spray of different plant growth regulators on increasing soybean seed weight.29–31 Moreover, Number of seed/10pod this traits all source of variations were no significant Number of main branches/plant was significantly, enhanced only at the non arid location by chemical treatments and significantly affected by cultivars and interaction between cultivars and treatments (Table 4) (Table 5). Results showed that Giza111 significantly branched more than Giza21.The obtained data at Abu Hummus reported that Lisophos 100ppm + MgSO4 1% (w/v) was the best with Giza 21 while MgSO4 1% (w/v) gave the highest value of branching with Giza111. Branching of soybean influences yield by providing plant with extra flowers and pods on branches32,33 reviewed that genetic variability in soybean branching varied among soybean genotypes, and that each country has developed soybean cultivars that generate the appropriate branch number in response to commonly used cultivation practices. The control of branch development is not the only soybean breeding strategy for increasing yield, but it remains an interesting topic in plant developmental biology. An increase in soybean branching with Lisophos and MgSO4 has never been reported yet but promising results of plant growth regulators and foliar spraying on increasing yield and its components in soybean34 might encourage researches concerning this topic. Length of main branch this characteristic was significantly influenced only by the applied treatments in Abu Hummus (Table 3) (Table 4). Each of Giza21 and Giza111 were taller at the non arid location than the arid location. El-Mohsen et al.,14 reported that Giza 111 and Giza 21 cultivars were taller than the other cultivars, since Giza was 111.50cm while Giza111 was 108.38cm. Stem Dry Weight was affected by the applied treatments, cultivar, and interaction among these factors at the two locations; except for cultivars at Abu Hummous location was non-significant for this trait (Table 3). In the arid location, the applied treatments, Lisophos 100ppm, Lisophos 50 ppm+KSO4 1% (w/v), KSO4 1% (w/v), MgSO4 1% (w/v) and Lisophos 100ppm + MgSO4 1% (w/v) resulted in increasing stem dry weight. While Lisophos 100 ppm at the non-arid location was the best and this concentration was also the best with Giza111.The growth habit of Glycine maxis either determinate or indeterminate. Determinate soybean plants stop vegetative growth and forming nodes on the main stem soon after flowering begins, whereas indeterminate varieties stay producing nodes on the main stem until the beginning of seed filling (growth stage R5)35–37 The increase in stem dry weight might be due to variations in stem vigor, taller plant and number of branches. Stem trans located the stored photo assimilates towards reproductive organs and for the growth of pods and grains in soybean38–40 and Masud,41 Rehenuma Tabassum et al.,42 Therefore plant with increased dry weight is expected to support seed filling and yield. Dry weight of seed/10pods (g). This parameter was significantly influenced by all sources of variance across the two locations except for cultivars at non arid location. KSO4 1% (w/v) gave the highest value for this trait at the non arid location, while Lisophos 100ppm + KSO4 1% (w/v) and MgSO4 1% (w/v) gave the highest value at the stressful location.
Potassium supports plant during its growth, as it increases its tolerance to drought; strengthen stem, and plant development.43 Pervious study carried out by Fehr & Caviness44 showed that a foliar spary with potassium sulfate at 18 to 36kg K ha−1 when soybean was at the V4 and R1-R2 stages of growth improved yield from 400 to 750 kg ha−1 compared to control. Several investigations45–47 observed an increase in the accumulation of N, P, K, and micronutrients in soybean tissues when K fertilizer was applied. Fresh weight pod/10 pods (g) Genotypes were significantly affected this traits at non-arid location and was insignificant within the arid location. The chemical treatments increased the weight of fresh weight/10 pods across the two locations. Genotypes by treatments were not significant across the two locations. Lisophos 50ppm showed best value for this trait compared to the other treatments in the non-arid location. On the other location all treatments were significantly differ than the control. Phospholipids homeostasis has an essential role at all plant growth stages.48 Apart from a main role in membrane structure and signal transduction events during morphogenesis, phosphor lipid metabolism is integral to embryo maturation, seed germination, auxin-stimulated cell division and growth, cell polarity, osmotic adjustment and stress tolerance, and delaying senescence49–53 reported that lysophospholipds especially Lysophosphatidylethanolamine (LPE) was able to delay leaf senescence which reflected on more carbohydrate partitioning from the source (mature leaves) to the sinks especially plant pods, whether under normal or arid condition in newly reclaimed areas.
In support of this-research findings, it was found that Lisophos (the commercial product of Lysophosphatidylethanolamine, LPE) is a natural compound that has been considered an effective growth regulator that initiated a new era of plant growth substances. This compound was able to retard leaf senescence while enhancing fruit coloration and keeping quality53–56 which means maintaining the plasma membrane integrity and keeping the leaves functional for longer duration during the season. Consequently, partitioning of carbohydrates from the mature leaves to various plant sinks is increasing such as the case with soybean pods that represent a strong sink. In addition, it was found that lisophos reduced electrolyte leakage of leaf57 and fruit tissue and enhanced its storability and shelf life58–60 It was the first inhibitor of the enzyme called phospholipase D (the senescence enzyme) as reported by many scientists.61 The ability to prolong the vase life of flowers62 provided another evidence for its ability to delay tissue senescence It also alleviated stresses of some pesticides or environmental conditions (Farag et al., 2003). LPE was able to avoid the adverse effects of ethephon on enhancing ripening of tomato without damaging the leaves.63 On the other hand, magnesium has been involved in chlorophyll biosynthesis, carbohydrate formation and increasing the rate of sugar pumping or exports from the source, (mature leaves) to various sinks in the plant such as berries, buds, branches, trunk and roots as well as pods in soybean. Potassium, on the other hand, causes an increase of the rate of carbohydrate biosynthesis in mature plant leaves.64–65
None.
The authors declared there is no conflict of interest.

©2019 Abouzied, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.