Advances in
eISSN: 2373-6402


Research Article Volume 8 Issue 6
Department of Botany, Aligarh Muslim University, India
Correspondence: Anushi Arjumend Jahan, Plant Biotechnology Laboratory, Department of Botany, Aligarh Muslim University, Aligarh- 202 002, India
Received: November 07, 2018 | Published: December 27, 2018
Citation: Jahan AA, Anis M. A short-term germplasm perpetuation protocol devised for Cardiospermum halicacabum L. using encapsulated microcuttings. Adv Plants Agric Res. 2018;8(6):607-610. DOI: 10.15406/apar.2018.08.00392
Synseed technology has been measured as the most expedient and cost-effective means for germplasm preservation under in vitro environment. It renders a system for storing tissues without physical and environmental stress. In the present study, nodal segments of Cardiospermum halicacabum L. were excised from proliferating in vitro shoot cultures developed from mature nodal explants and encapsulated best in 3% (w/v) sodium alginate complex with 100 mM CaCl2.2H2O. The maximum conversion rate was achieved in MS medium augmented with 2- iP (5.0μM) producing a maximum of 2.67±0.88 numbers of shoots after 8 weeks of culture. The utmost rate of recurrence of plantlets from encapsulated nodal segments stored at 4°C was attained after 4 weeks with 90% conversion competence. Plantlets obtained from synseeds were hardened, established successfully ex vitro and were morphologically similar to each other as well as their mother plant.
Keywords:artificial seed, cold storage, calcium chloride, conversion, clonal propagation, sodium alginate
2-iP, 2-Isopentenyl adenine; BA, 6-Benzyladenine; Kn, Kinetin; MS, Murashige; Skoog medium
C. halicacabum L. (Sapindaceae), generally identified as balloon vine, is a valuable medicinal climber, extensively stretched in tropical and sub-tropical regions of the world. Ethnobotanically, the plant is harvested in backyards for both food and medicinal value. The herb is diuretic, stomachic, antispasmodic and rubefacient. It is used in rheumatism, nervous diseases and reduces irritation and inflammation of skin.1 The pharmacological studies on Cardiospermum halicacabum have revealed the presence of flavonoids such as apigenin, pinitol, luteolin which are reported to possess the antidiabetic, analgesic, anti-inflammatory, antifilarial, antibacterial, anti-diarrheal, antipyretic, anti parasitic, anti malarial and antioxidant activities.2‒8 Traditionally, the plant is proliferated all the way through seeds but this is not sufficient to meet the increasing demands due to low germination pace (35-40%), little viability and deferred rooting of seedlings.9 Furthermore, large scale unrestricted utilization of intact plant to match its ever-increasing demand by the pharmaceutical business, attached with limited nurturing and unsatisfactory efforts for its replenishment, the natural population of this valuable medicinal plant have been noticeably washed-out. Despite possessing such immense medicinal properties, there are only few reports available on in vitro regeneration and propagation using different explants of Cardiospermum halicacabum.10 But, there is no information available on the synthetic seed creation in balloon vine using vegetative propagules.
Conservation of plant germplasm by in vitro technology has also been done using one of the promising ventures i.e., the Synthetic Seed Technology. This scheme has acknowledged momentous attention as an impending cost effective clonal propagation system straightening out a new outlook to plant biotechnology. The technique of synthetic seeds is one of the quick means of plant regeneration because of its extensive use in preservation and rescue of regenerated plants.11 Previously, synseeds was known to be an encapsulated somatic embryo but now, other micropropagules like shoot buds, shoot tips, nodal explants, organogenic or embryogenic calli or unipolar structures have been engaged in the manufactuing of synseeds. There are few reports available which encapsulates vegetative propagules like axillary or apical buds which may well be used for clonal propagation as well as long term conservation of germplasms.12‒14
To the best of our acquaintance, this protocol presents primary information on the development of an efficient conservation protocol to store high-yielding C. halicacabum genotypes at low temperature by means of synthetic seed technology using nodal segments from axenic shoot cultures of mature plant.
Plant material and explant source
Nodal explants were cut out from 8 weeks old in vitro cultures of C. halicacabum build up from 4 years old adult plant from the Botany Department, Aligarh Muslim University, Aligarh, India. The 8 weeks old in vitro cultures were nurtured on the procedure developed via Jahan & Anis.10
Encapsulation
Sodium alginate (Qualigens, India) at diverse concentrations (1, 2, 3, 4 and 5% (w/v)) was supplemented to liquid MS medium. For complexation, varying levels of CaCl2.2H2O solution prepared in liquid MS medium is used (25, 50, 75, 100 and 200 mM). The pH of the gel matrix and the complexing agent was adjusted to 5.8 prior to autoclaving at 121°C for 20 min. Encapsulation was done by integrating nodal explants in sodium alginate solution using a pipette and dropping them in CaCl2.2H2O solution. The droplets enclosing the explants were held atleast for 20 min. to attain polymerization of the sodium alginate. Now, these calcium alginate beads containing the nodal segments were rescued from the solution and washed two times with autoclaved distilled water to get rid of the any traces of CaCl2.2H2O and then shifted to disinfected filter paper in petridishes under the laminar airflow cabinet to eradicate the surplus of water and after that planted onto petridishes containing nutrient medium.
Shoot regeneration and culture conditions
The calcium alginate beads possessing nodal segments were moved to wide mouth culture flask (Borosil, India) including MS medium complemented with plant growth regulators BA, Kn or 2-iP at different concentrations (2.5µM, 5.0µM and 10.0µM). The culture medium was solidified with 0.8% (w/v) agar and pH was adjusted to 5.8 prior to autoclaving at 121°C for 20 min. Cultures were maintained at 24±2°C under 16/8 h light-dark conditions with a PPFD of 50μmolm-2s-1 provided by cool white fluorescent tubes.
Low temperature storage
A set of encapsulated nodal beads were transferred to water-agar medium and stock up in refrigerator at 4°C. Seven diverse low temperature revelation times (0, 1, 2, 3, 4, 5 and 6 weeks) were assessed for regeneration. Following every storage phase, encapsulated nodal segments were cultured on MS medium supplemented with plant growth regulators for conversion into plantlets. The percentage of encapsulated nodal segments forming shoots and roots were recorded after 8 weeks of culture to regeneration medium. The plantlets developed from encapsulated nodal segments were hardened off and acclimatized as specified below.
Acclimatization
In vitro raised rooted plantlets were taken away from the culture medium, washed gently with tap water and transferred to thermocol cups containing sterile SoilriteTM (Keltech Energies Ltd., Bangalore, India), watered with half- strength MS salt solution deficient with organic nutrients and kept under diffused light (16/8 h photoperiod) conditions. Potted plantlets were sheltered with a crystal clear polythene bags to retain high humidity and irrigated after every week. The polybags were removed after 2 weeks in order to acclimatize plants to field conditions. After 4 weeks, properly hardened plants were transferred to pots containing normal garden soil and vermiculite mixture in the ratio of 1:1and finally transferred to field conditions.15
Statistical analysis
Each conducted experiment was done with a minimum of ten replicates per treatment and continued three times. The data were examined statistically using SPSS Ver.10 (SPSS Inc, Chicago, USA). The significances of differences among means was carried out using Duncan’s multiple range test at P=0.05. The results are expressed as a means±SE of three repeated experiments.
Outcome of alginate concentration on bead creation
Nodal explants are the most appropriate explants for synseed preparation as they were exceptionally best plant material because these explants with pre-existing meristematic tissues ensures the genetic stability of regenerants. Micropropagation through encapsulation of vegetative propagules has also been detailed previously in many medicinal plant species like Withania somnifera,16 Tylophora indica,17 Rauvolfia serpentina,18 Spilanthus acmella,19 Eclipta alba,20 Balanites aegyptiaca.14
In the present study, nodal segments from axenic shoot cultures of Cardiospermum halicacabum produced from mature nodal explants of mother plant through the protocol of Jahan & Anis10 were used for encapsulation. An unbeaten proliferation scheme is founded on major assessment of factors affecting the gel matrix and both sodium alginate and CaCl2.2H2O plays a vital role in complexation and capsule quality.21 Here, the bead formation was influenced by different concentrations and combinations of sodium alginate (1, 2, 3, 4 and 5%(w/v)) and CaCl2.2H2O (25, 50 75, 100 and 200 mM). A 3% solution of sodium alginate upon complexation with 100 mM CaCl2.2H2O produced firm, clear, uniform beads within ion exchange duration of 30 min (Figure 1A). Lower concentrations of sodium alginate and calcium chloride resulted in soft beads which are too delicate to handle while elevated levels of both resulted in hard beads which causes considerable delay in the shoot emergence. Gel encapsulation using sodium alginate and calcium salt was a valuable method for encapsulation as this is a very excellent amalgamation since the ions are non-damaging, easy to use, have a low price and propagule to plant conversion occurs successfully.22
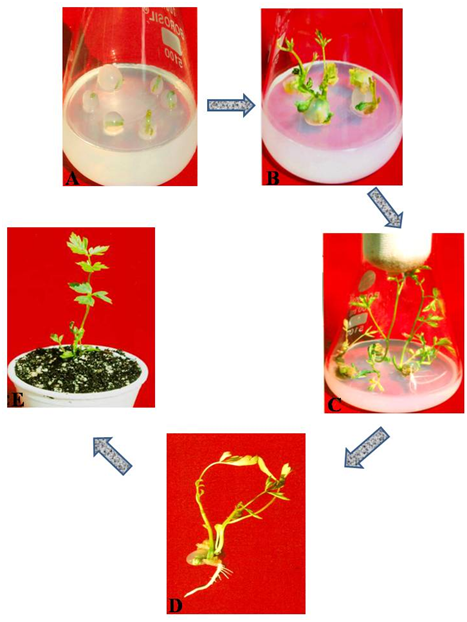
Figure 1
Plantlet regeneration from encapsulated nodal beads
Nodal segments encapsulated in 3% sodium alginate and 100 mM CaCl2.2H2O showed evidence of shoot regeneration after 2-3 weeks of culture on MS medium amended with BA, Kn or 2-iP (2.5μM, 5.0μM and 10.0μM). These encapsulated nodal segments of Cardiospermum halicacabum exhibited the best conversion (70%) into whole plantlets after 8 weeks of culture on MS medium amended with 2-iP (5.0 μM) generating a highest number of shoots (2.67±0.88) as depicted from Table 1, Figure 1(A–C). The reason for these results may be attributed to the protection provided by the capsules plus the presence of nutrients in the gel matrix, which seems to serve as a nutrient cover around the encapsulated buds, making promising growth, endurance and germination. This is in harmony to the discovery of Naik & Chand,23 Faisal & Anis,24 Ahmad & Anis,25 Sharma & Shahzad,13 Ahmad et al.12 and Jahan & Anis.26
|
Treatments into plantlets |
%Conversion response of shoots |
Mean number |
|
MS + BA (2.5μM) |
10 |
1.00±0.00b |
|
MS + BA (5.0μM) |
13 |
1.33±0.33ab |
|
MS + BA (10.0μM) |
10 |
0.66±0.33b |
|
MS + Kn (2.5μM) |
20 |
1.67±0.33ab |
|
MS + Kn (5.0μM) |
27 |
1.33±0.33ab |
|
MS + Kn (10.0μM) |
16 |
1.00±0.00b |
|
MS + 2-iP (2.5μM) |
40 |
2.00±0.57ab |
|
MS + 2-iP (5.0μM) |
70 |
2.67±0.88a |
|
MS + 2-iP (10.0μM) |
60 |
1.66±0.33ab |
Table 1 Effect of different media on conversion of encapsulated nodal segments of Cardiospermum halicacabum after 8 weeks of culture on MS medium
Values represent means±SE. Means followed by the same letter within columns are not significantly different (P=0.05) using Duncan’s multiple range test.
Low temperature storage
An essential characteristic of the encapsulated vegetative propagules is their ability to retain viability after storage for an adequate phase compulsory for exchange of germplasm between laboratories and extension centers.27 The translation of encapsulated nodal beads into complete plantlets after substantial time of storage could be credited to the encapsulation of MS nutrients in encapsulation medium which serves as a synthetic endosperm to the encapsulated explants for regeneration.28 In the present attempt, the encapsulated nodal beads of Cardiospermum were stored for 1, 2, 3, 4, 5 and 6 weeks at 4°C to assess the effect of cold storage on the regeneration status of explants (Table 2). The synthetic seeds stored at 4°C for a period of 4 weeks resulted in maximum conversion frequency (90%) within 8 weeks of culture on MS medium supplemented w ith 2-iP (5.0μM) as evident from Table 2. However, as storage period increased beyond 4 weeks, a decline in conversion response was noticed. This turn down in the frequency of retrieval of plantlets from encapsulated nodal explants stored at low temperature is because of inhibition in respiration process of plant tissues due to alginate cover. Similarly, the conversion ability of encapsulated nodal segments of Punica granatum L. also declined markedly following storage at low temperature.22,23 The data of the present investigation with cold stored encapsulated nodal segments of Cardiospermum halicacabum are in accordance with those of Faisal et al.,29 Ahmad et al.,12 Sharma & Shahzad13 and Varshney & Anis14 regarding the rates of conversion from encapsulated axillary buds in Rauvolfia serpentina, Ruta graveolens, Decalepis hamiltonii and Balanites aegyptiaca respectively.30,31
|
Storage duration into plantlets |
% Conversion response of shoots |
Mean number |
|
0 |
70 |
1.33±0.33ab |
|
1 |
73 |
1.00±0.00b |
|
2 |
80 |
1.00±0.00b |
|
3 |
86 |
1.66±0.33ab |
|
4 |
90 |
2.00±0.00a |
|
5 |
77 |
1.33±0.33b |
|
6 |
50 |
1.00±0.00b |
Table 2 Effect of storage duration at 4°C on conversion of encapsulated nodal buds of Cardiospermum halicacabum after 8 weeks of culture
Rooted plantlets (Figure 1D) with full stretched leaves, retrieved from encapsulated nodal segments were shifted to thermocups full of disinfected soilrite and sheltered with transparent polythene bags inside growth chamber for 4 weeks (Figure 1E). After one month, these were transferred to earthen pots containing a mixture of garden soil and vermiculite and maintained in greenhouse where they grew normally.
By devising the present protocol, better and cloned plants of Cardiospermum halicacabum could be obtained. In addition, preservation of this plant species would help in extending biodiversity with germplasm conservation and helps in exchange of germplasm between countries. Also, cold storage has the potential to reduce the cost of maintaining germplasm cultures because of the need for manual labor due to less frequent subculturing.
Research support from the Department of Science and Technology (Govt. of India) New Delhi under the DST-FIST (2011) and UGC-SAP (2009) Programmes are duly acknowledged.
Author declares that there is none of the conflicts.

©2018 Jahan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.