Advances in
eISSN: 2373-6402


The antimicrobial potential of eight selected medicinal plants was evaluated against medically important thirteen pathogenic bacteria and three pathogenic fungi by using disc diffusion method. Moreover, the free radical scavenging activity was investigated for the development of anti-aging ingredient as a raw product for the cosmetic and other industries. Petroleum ether, ethyl acetate, methanol, butanol, n-hexane water extracts of medicinal plants were used for antimicrobial and anti-oxidant investigation. The rapid evaluation of antioxidant activity of different crude extracts of Pouzolzia zeylanica, Equisetum debile, Memecylon umbellatum, Datura metel, Cauroupeta guianensis, Andrographis paniculata were determined by using DPPH free radical method. The microbial inhibition zone ranged from 7-15 mm for different extracts of Andrographis paniculata where ethyl acetate extract showed highest microbial inhibition. The petroleum ether extract of D. metel gave 9-10 mm microbial inhibition except Pseudomonas aureus. Aloe vera gave 7-9 mm inhibition against microorganism by ethyl acetate extract. The methanol extract of A. paniculata showed inhibition only against two gram-positive and five gram-negative bacteria. The methanol extract of P. zeylanica, M. umbellatum and butanol (n-BuOH) extract of A. vasica also showed different range of antimicrobial activity. The N-hexane extract of A. vera and A. vasica and methanol extract of Equisetum debile did not show any antimicrobial activity. These extracts resulted in a rapid increase and decrease of the absorbance and showed different hydrogen-donating capacity towards the 2, 2′-diphenyl-1-picrylhydrazyl (DPPH) radical. A lot of differences found and showing anti-oxidant activity of different solvent extracts of different plant species. Among the species, ethyl acetate extract of E. debile, M. umbellatum, methanol Extract of M. umbellatum, C. guianensis and n-BuOH extract of M. umbellatum, C. guianensis showed the maximum scavenging capacity of over 80.
Keywords: antioxidants, antimicrobial agents, DPPH, medicinal plants, phytomedicine, reactive oxygen species
DPPH, diphenyl picryl hydrazyl; BCSIR, bangladesh council of scientific and industrial research; INFS, institute of nutrition and food science; DPPH, 2, 2-diphenyl-1-picrylhydrazyl; PE, petroleum ether; EA, ethyle acetate; MeOH, methanol; BuOH, butanol
Herbal medicine is widely used in Bangladesh in traditional healthcare system such as Ayurvedic, Unani, Hekimi and other form of folk treatments.1 Almost 80% of rural population is dependent on medicinal plants for their primary health care. Now medicinal plants are gaining more importance in pharmaceutical industries for the preparation of new phytomedicines.1 Antimicrobial screening is the first stage of antimicrobial drug research to ascertain the susceptibility of pathogenic microorganisms to any plant agent. Herbal industries always use the crude extracts of medicinal plants for their medicine and beautifying constituents. In most cases, they use medicinal plant crude extract without confirming or validating their level of toxicity and active ingredient. However, it is necessary to know which compound is specifically responsible for showing specific activity.
There are some common local plants those may have significant antibiotic activity which are commonly used in herbal industries in the country. Therefore, it is necessary to screen those plants against microorganisms that cause human infection. This antimicrobial ability of plant extract estimated by any of the following three methods; viz. disc diffusion method, serial dilution method and bio-autographic method. But there is no standardized method for antimicrobial screening.3 Some investigators used the diameter of zone of inhibition and/or the minimum weight of extract to inhibit the growth of microorganisms. However, a great number of factors viz., the extraction methods, inoculums volume, culture medium composition, pH and incubation temperature can influence the results.
There is an increasing interest in antioxidants, particularly in those intended to prevent the presumed deleterious effects of free radicals in the human body, and to prevent the deterioration of fats and other constituents of foodstuffs. In both cases, there is a preference for antioxidants from natural rather than from synthetic sources.4 There is therefore a parallel increase in the use of methods for estimating the efficiency of such substances as Antioxidants5,6 because these compounds in food play an important role as a health-protecting factor and the huge number of medicinal plants also contains the antioxidant compound for investigation. One such method that is currently popular is based upon the use of the stable free radical diphenyl picryl hydrazyl (DPPH). Several plant species have already been reported for their antioxidant activities in human body. We used number of medicinal plants of Bangladesh in this experiment (Figure 1), for instance, Couroupita guianensis bark leaves and flowers have been used traditionally for medicinal purposes. It is said to have anti-bacterial, antiseptic and analgesic qualities, the bark supposedly cures colds, the juice from its leaves is good for treating malaria and for skin diseases, while chewing young leaves alleviates toothache, and the interior of the fruit can disinfect wounds.7-10 Varied species of Datura have been used in traditional medicine worldwide, primary among them Datura innoxia, D. metel, and D. stramonium. The primary use of Datura is as a hallucinogenic and intoxicant, though it does have medicinal uses. The most common medicinal uses for Datura in these systems are for skin conditions, anxiety disorders, and respiratory ailments, along with a litany of other conditions. The seeds are also sometimes used as a substitute for opium.11 They are useful to control the root knot nematode Meloidogyne javanica in crop plants. Previous phytochemical investigation of the plant revealed the presence of chemical constituents such as flavones, flavonoids, tannin, carotene, carotenoids, ascorbic, tartaric, malic and pectic acids, gum, minerals and their salts.1 Antimicrobial and antioxidant properties in the leaves of Memecylon umbellatum Burm., have been used to treat many diseases and physiological disorders like leucorrhoea and gonorrhea.12-15 Wound healing activity of ethanolic extract of the leaves has also been reported.16 Equisetum debile is administered as a cooling medicine which is well known to be used for the remediation of nasal polypus, various cancers of breast, liver, intestine, stomach, kidneys and tongue.17 Pouzolzia zeylanica (L.) (Graceful Pouzolzbush; Urticaceae) is extensively grown in Bangladesh. The Leaves are anthelmintic and vulnerary; used as a cicatrizant for gangrenous ulcers, in syphilis and gonorrhoea. Leaf juice is used as galactagogue. Poultice of the herb is applied to sores, boils and to relieve stomachache.16 Previous phytochemical investigation of the plant revealed the presence of chemical constituents such as flavones, flavonoids, tannin, carotene, carotenoids, ascorbic, tartaric, malic and pectic acids, gum, minerals and their salts.1
Kalmegh (Andrographis paniculata Nees, king of bitter or Moha-tita family: Acanthaceae) is an important and well known medicinal plant in the world. It is widely cultivated and grows abundantly in southeastern Asia: India, Srilanka, Bangladesh, Pakistan and Indonesia. In Bangladesh, there are more than 20 species and 8 varieties of the genus Andrographis available17 including A. paniculata. Only a few species are medicinal, of which A. paniculata is the most popular. Since ancient times, Kalmegh is used in traditional Siddha and Ayurvedic systems of medicine as well as in tribal medicine in India and some other countries for multiple clinical applications. Andrographolide is also attributed with such other activities like liver protection. Several studies have been conducted on the cellular processes and targets modulated by Andrographolide treatment that inhibited the proliferation of different tumor cell lines, representing various types of cancers. In one Chilean study, the herb had a significant drying effect on the nasal secretions sufferers who took 1,200 milligrams of Andrographis extract daily for five days.18
The Aloe vera gels stimulate cell growth and restore damaged skin. It is widely used as moisturizing the skin because of its water holding capacity. Aloe vera juice has been considered as the factor of relieving many gastrointestinal irritations and may protect the mucous membrane of the stomach.19 In many countries, like Germany, United States, concentrated extracts of dried Aloe leaves are used as laxative preceding, rectal surgery and as a hemorrhoid treatment. It is also prescribed to relieve thermal burn, sunburn and wound healing 19 and a lot of researches suggest that Aloe gel can enhance immune system of the body.20
Evergreen Hebaceous Basok (Adhatoda vasica Nees., Acanthaceae) is a medicinal plant native to Asia, found abundantly in wild in all over Nepal, India, Pakistan and Bangladesh. Several alkaloids of Adhatoda found to posses antispasmodic properties. The plant is also used against various chest ailments. The plant causes a slight inhibition of spontaneous movements of nerve muscle. The constituents have the potentiality of broncho-dilating activity and the oil also showed anti-insects and juvenile hormone mimicking activity.21,22
Scientific evidence suggests that antioxidants reduce risk for chronic diseases including cancer and heart disease. Most of the antioxidant compounds in a typical diet are derived from plant sources and belong to various classes of compounds with a wide variety of physical and chemical properties. The main characteristic of an antioxidant is its ability to trap free radicals. Highly reactive free radicals and oxygen species are present in biological systems from a wide variety of sources. The use of DPPH for a radical scavenging measuring method is described by Vinothapooshan & Sundar, Claeson et al., Masuda et al.23-25 because of its stability in a methanolic solution. The DPPH method is described as a simple, rapid and convenient method independent of sample polarity for screening of many samples for radical scavenging activity.26 Considering these facts, this study was carried out to investigate the antimicrobial activity against some common human pathogenic microorganisms and to find out the free radical scavenging activity of antioxidants in different solvent crude extracts of some important medicinal plants material.
The experiment was conducted at Sher-e-Bangla Agricultural University, Dhaka, Bangladesh and Bangladesh Council of Scientific and Industrial Research (BCSIR) laboratory during 2013 to 2014.
Test plant materials
Fresh samples were collected from the Medicinal germplasm center of Sher-e-Bangla Agricultural University, Dhaka, Bangladesh. Flowers of cannon ball (Couroupita guianensis), aerial stem of horsetail (Equisetum debile), leaf of Iron-wood tree (Memecylon umbellatum), leaf of graceful Pouzol bush (Pouzolzia zeylanica), seed of Indian thorn apple (Datura metel), fresh leaf gel of Aloe vera and leaf of Kalmegh (Andrographis paniculata) were taken for the investigation. The details of tested plant materials are presented in Table 1.
Name of plant |
|
||||
Local name |
Common name |
Botanical name |
Family |
Plant parts used |
|
Kalmegh |
King of bitters |
Andrographis paniculata |
Acanthaceae |
Leaf |
|
Naglingom |
Cannon Ball |
Couroupita guianensis |
Lecythidaceae |
Flower |
|
Basak |
Malabar Nut, Adulsa |
Adhatoda vasica |
Acanthaceae |
Leaf |
|
Ghritokumari |
Aloe |
Aloe vera |
Xanthorrhoeaceae |
Leaf |
|
Dhutra |
Devil's trumpet |
Datura metel |
Solanaceae |
Seed |
|
Kallurki |
Graceful pouzolzsbush |
Pouzolzia zeylanica |
Urticaceae |
Leaf |
|
Anjani, Alli |
Iron wood |
Memecylon umbellatum |
Melastomataceae |
Leaf |
|
Sumbak, Sime Jhar |
Horsetail, snake grass |
Equisetum debile |
Equisetaceae |
Stem |
|
Table 1 Selected medicinal plants used in the experiment
Preparation of the plant extracts
Firstly the samples were dried below 25°C at room temperature for 7days. Then the samples were blended by a blender machine (Moulinex, France). Dried powders of different treatments were dissolved in sample bottles with different solvents for 3days. Each day each bottle was shacked well and filtered with a filter paper (Millipore, Germany). The filtrate was then evaporated by a rotary vacuum evaporator (Buchi, England). This procedure was repeated for 3-5times until the total compounds separated from the powder. All evaporations were carried out under reduced pressure using a rotary evaporator at a bath temperature of 45°C. The residual solvent of the extracts were transferred to a tube (Eppendorf, Germany) for testing antimicrobial activity by disc diffusion method.27
Test organisms
Clinical isolates of the microorganisms were used for antimicrobial test (Table 2). The pathogenic bacterial and fungal pure cultures were collected from the Institute of Nutrition and Food Science (INFS), University of Dhaka, Bangladesh. Both Gram positive and Gram-negative organisms were taken in this experiment.
Preparation of medium and sub-culture
To prepare required volume of this medium, calculated amount of each of the constituents (DIFCO, India; Bactopeptone-0.5g, Bacto yeast extract -1.0g, Nacl-0.5g, Bacto agar-2.0g, Distilled water-100ml) was taken in a conical flask and distilled water was added to it to make the required volume. The contents were heated in a water bath to make a clear solution. The pH (at 25°C) was adjusted at 7.2–7.6 using NaOH or HCl. 10ml and 5ml of the medium was then transferred in screw cap test tubes to prepare plates and slants respectively. The test tubes were then capped and sterilized by autoclaving at 15-lbs pressuresq.-1 inch at 121°C for 20minutes. The slants were used for making fresh culture of bacteria and fungi that were in turn used for sensitivity study. In order to avoid any type of contamination and cross contamination by the test organisms the antimicrobial screening was done in Laminar Hood and all types of precautions were highly maintained. UV light was switched on one hour before working in the Laminar Hood. Petri dishes and other glassware were sterilized by autoclaving at a temperature of 121°C and a pressure of 15-lbssq-1 inch for 20min. In an aseptic condition under laminar air cabinet, the test organisms were transferred from the pure cultures to the agar slants with the help of a transfer loop to have fresh pure cultures. The inoculated strains were then incubated for 24hours at 37 °C for their optimum growth. These fresh cultures were used for the sensitivity test.
Preparation of the test plates
The test organisms were transferred from the subculture to the test tubes containing about 10ml of melted and sterilized agar medium with the help of a sterilized transfer loop in an aseptic area. The test tubes were shaken by rotation to get a uniform suspension of the organisms. The bacterial and fungal suspension was immediately transferred to the sterilized petri-dishes. The petri-dishes were rotated several times clockwise and anticlockwise to assure homogenous distribution of the test organisms in the media.
Preparation of sample discs with test samples
Test samples were dissolved in solvent to obtain the desired concentrations in an aseptic condition. Sterilized metrical filter paper discs (BBL, Cocksville, USA) were taken in a blank petridish and were soaked with solutions of test samples and dried. The amount of sample per disc was 500µl. The sample discs, standard antibiotic discs and control discs were placed gently on the previously marked zones in the agar plates pre-inoculated with test bacteria and fungi. The plates were then kept in a refrigerator at 4°C for about 24h upside down to allow sufficient diffusion of the materials from the discs to the surrounding agar medium. The plates were then inverted and kept in an incubator at 37°C for 24h.
Antimicrobial activity measurements by the zone of inhibition
The antimicrobial potency of the test materials were measured by disc diffusion method where the plant extract activity prevent the growth of the micro-organisms surrounding the discs which gives clear zone of inhibition.13,14 After incubation, the antimicrobial activities of the test materials were determined by measuring the diameter of the zones of inhibition in millimeter (mm) with a transparent scale.
Free Radical Scavenging Activity Test (DPPH Method)
The free radical scavenging activity was assayed spectrophotometrically.28 The DPPH (2, 2-Diphenyl-1-picrylhydrazyl) radical has a deep violet color due to its unpaired electron, and radical scavenging activity can be followed by absorbance at 525 nm Sample stock solutions (1mg ml-1) were diluted to final concentrations g ml-1 in 70% ethanol or DMSO. The DPPH ethanolmof 100, 50, 10 and 5 solution (0.2mM, 0.5ml) was added to 1ml of sample solutions of different concentrations, shaken well by vortex, and allowed to react at room temperature. The absorbance values were measured after 10min at 525nm by UV/V is spectrophotometer.
The free radical scavenging activity of samples was calculated according to the formula.29
DPPH radical scavenging activity (%) =[1−(Abssample −Absblank)/Abscontrol]×100
Where, Abssample is the absorbance of the experimental sample, Absblank is the absorbance of the blank; Abscontrol is the absorbance of the control (vitamin-C).
As a blank, 70% EtOH or DMSO solvent (0.5ml) and sample solution (1.0ml) were used. DPPH solution (0.5ml, 0.2mM) & 70% EtOH or DMSO solvent (1.0ml) was used as a negative control. The ascorbic acid (vitamin C) was used as a positive control. Each treatment was replicated twice. Antioxidant activity was expressed as the concentration of each sample that decreased the radicals by half (SC50, 50% scavenging capacity).
It has long been recognized that naturally occurring substances in higher plants have antimicrobial and antioxidant activity. Among the available methods, disc diffusion method for antimicrobial assay and on the other hand, the compounds distributed in plants have the ability to scavenge free radicals by single-electron transfer. In our experiments, we measured the anti-microbial and total antioxidative effect of some medicinal plants. Because of the complex nature of phytochemicals, the antioxidant and antimicrobial activities of plant extracts should be evaluated separately. The results of this experiment would be encouraged in future to the application of these medicinal plants in pharmaceutical and cosmetic formulations.
Antimicrobial activity analysis of different crude extracts
Result obtained from this study revealed that, the crude petroleum ether (PE) extracts of Datura seed showed a maximum inhibition activity against four gram (+) and three gram (-) and also for the three fungi (Table 2). Besides, PE and also MeOH extracts of Cauroupita flower powder showed a significant activity for three gram (+) and four gram (-) bacteria and moderate activity for other organisms, where the standard disc was doxycillin (30µg disc-1). The crude MeOH extract of the dry leaves of P. zeylanica, M. umbellatum showed moderate inhibition activity against five gram positive, eight gram negative bacteria and three fungi except Sarcina lutea but MeOH extract of Equisetum debile showed lower antimicrobial activity against them by disc diffusion method, where Kanamycin 30µg disc-1 disc was used as a standard. The antimicrobial activity of crude MeOH extract of A. paniculata dry leaves showed maximum activity against Salmonella typhi (10 mm) and Bacillus subtilis where the crop was cultivated with organic fertilizer, is an indication that the leaf extract is effective for pharmacological test and can be beneficial as a cure for different diseases like fever and skin diseases.
Gram-positive bacteria |
Gram-negative bacteria |
Fungi |
Bacillus Sereus |
Escherichia coli |
Candida albicans |
Bacillus Megaterium |
Pseudomonas aureus |
Aspergillus niger |
Bacillus Subtilis |
Salmonella paratyphi |
Sacharomyces cerevacae |
Sarcina Lutea |
Salmonella typhi |
|
Staphylococcus Aureus |
Shigella boydii |
|
Shigella dysenteriae |
||
Vibrio mimicus |
||
Vibrio parahemolyticus |
Table 2 Tested microorganisms used in the experiment
Cannon ball (C. guianensis) flower powder was tested with petroleum ether (PE), ethyle acetate (EA), methanol (MeOH) and butanol (BuOH) crude solvents. The zone of inhibition (mm) by gram (+), gram (-) and fungi were different but varied significantly in different solvents. The maximum inhibitory activity of PE was 10±1mm by gram positive Bacillus, gram negative Pseudomonas, Salmonella and or other organisms (Table 3). The antimicrobial activity measured by following a scale of >10mm–excellent, 7-10mm- moderate to good and <6mm- lower activity. EA extract showed an excellent inhibition activity (15mm) found by gram positive Bacillus bacteria and the moderate activity (13mm) showed by Pseudomonas sp., Salmonella typhi, Vibrio spp. and Shigella spp. that might be an indication of for severely immune-compromised patients and food poisoning30–32 animal feed33 and for fried rice syndrome.34–36 The MeOH extracts also showed an influential activity (10±1mm) to the organisms taken for the investigation.
The moderate to good zone of inhibition exhibited by ethyl acetate (EE) leaf gel extract of Aloe vera against almost all tested pathogenic microorganisms, having the zone of inhibition of 8±1 mm each (Table 3). However, the n-hexane extracts did not show any activity against the bacteria, fungi and yeast. The microbial activity was remarkable in the seeds of D. metel as zone of inhibition was found in the PE-Datura seed extract against all microorganisms (Table 3). Butanol and n-hexane extract of Adhathoda were tested for antibacterial and antifungal activity where standard disc of amoxicillin (30mg disc-1) was used for comparison purpose. The butanol extracts of A. vasica leaves showed antimicrobial activity in terms of zone of inhibition (7-8mm) at a concentration of 400mg disc-1 (Table 3). On the other hand, n-hexane extracts did not show any activity against tested microorganisms. The antimicrobial activity of MeOH extract of M. umbellatum was determined against tested microorganisms by disc diffusion method, where Kanamycin 30 (µg disc-1) disc was used as a standard. The results showed moderate antimicrobial activity (7-10 mm) against all organisms except Sarcina lutea (Table 3). The best antimicrobial activity was noticed against Bacillus megaterium.
The crude MeOH leaf extract of P. zeylanica and M. umbellatum showed moderate antimicrobial activity against tested microorganisms. However, MeOH extract of E. debile did not show antimicrobial activity (Table 3). The MeOH extract of A. paniculata leaf showed notable activity against Salmonella typhi (10mm). Bacillus subtilis showed antimicrobial activity (9mm) that supported by Maneemegala & Naveen37 Bacillus megaterium also showed similar results (7mm). Salmonella paratyphi, Vibrio spp., and Shigella dysenteriae produced different level of inhibition zone (Table 3). A. paniculata did not show any antimicrobial activity to the rest of the organisms.
Name of extract |
Name of plant |
Inhibition Zone (mm) Against* |
|||||||||||||||
|
|
BSe |
BM |
BS |
SA |
SL |
EC |
PA |
SP |
ST |
VM |
VP |
SD |
SB |
CA |
AN |
SC |
Doxycillin (Standard disc) ( 30 mg/disc) |
|
50 |
50 |
50 |
50 |
50 |
50 |
50 |
50 |
49 |
50 |
49 |
50 |
49 |
50 |
50 |
50 |
Petroleum Ether |
C. Guianensis |
10 |
11 |
10 |
9 |
9 |
9 |
10 |
9 |
10 |
9 |
9 |
9 |
9 |
9 |
9 |
9 |
|
D. Metel |
10 |
10 |
10 |
9 |
10 |
9 |
0 |
10 |
10 |
9 |
9 |
9 |
10 |
10 |
10 |
10 |
Ethyl Acetate |
C. Guianensis |
15 |
15 |
15 |
12 |
12 |
12 |
13 |
12 |
13 |
13 |
12 |
13 |
12 |
12 |
12 |
12 |
|
A. Vera |
9 |
9 |
8 |
8 |
7 |
8 |
7 |
8 |
8 |
8 |
8 |
8 |
8 |
8 |
8 |
8 |
Methanol |
C. Guianensis |
11 |
10 |
10 |
9 |
10 |
10 |
10 |
10 |
10 |
9 |
9 |
10 |
10 |
10 |
10 |
10 |
|
A. Paniculata |
0 |
7 |
9 |
0 |
0 |
0 |
0 |
8 |
10 |
8 |
8 |
7 |
0 |
0 |
0 |
0 |
Butanol |
C. Guianensis |
9 |
9 |
9 |
8 |
8 |
7 |
8 |
8 |
8 |
8 |
7 |
7 |
8 |
8 |
8 |
8 |
|
A. Vasica |
8 |
8 |
8 |
8 |
7 |
8 |
8 |
8 |
8 |
7 |
7 |
8 |
8 |
8 |
8 |
8 |
n - Hexane |
A. Vera |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
A. Vasica |
7 |
7 |
7 |
9 |
7 |
9 |
7 |
7 |
7 |
7 |
9 |
7 |
7 |
6 |
6 |
6 |
Kanamycin (Standard disc) (30 µg disc-1) |
|
32 |
31 |
31 |
31 |
31 |
32 |
32 |
32 |
32 |
32 |
34 |
31 |
32 |
32 |
31 |
31 |
Methanol |
P. Zeylanica |
10 |
9 |
8 |
8 |
7 |
8 |
8 |
8 |
8 |
10 |
9 |
9 |
0 |
7 |
9 |
7 |
|
E. Debile |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
M. Umbellatum |
7 |
10 |
8 |
0 |
7 |
8 |
9 |
9 |
7 |
7 |
7 |
8 |
7 |
7 |
8 |
7 |
Table 3 Antimicrobial activity of different extracts of medicinal plants against human pathogenic bacteria and fungi by using disc diffusion method
Two different standard discs were used on the basis of availability. >10mm – excellent, 7-10mm- moderate to good and <6mm- lower activity.
*BSe, bacillus sereus; BM, bacillus megaterium; BS, bacillus subtilis; SA, staphylococcus aureus; SL, sarcina lutea; EC, escherichia coli; PA, Pseudomonas aureus; SP, salmonella paratyphi; ST, salmonella typhi; VM, vibrio mimicus; VP, vibrio parahemolyticus; SD, shigella dysenteriae; SB, shigella boydii; CA, candida albicans; AN, aspergillus niger; SC, sacharomyces cerevacae
Antioxidant analysis of different crude extracts of medicinal plant samples
For the verification of scavenging activity, the scavenging capacities of tested samples were measured spectrophotometrically with DPPH (free radical) that create a violet solution in ethanol. A reduced antioxidant molecule, give no color, which has been used to evaluate the antioxidant activity of plant and microbial extracts.36–39 Each of the plant extract was examined using stable DPPH radicals, all the used extracts exhibited the various scavenging capacity. The free radical scavenging activity of the seventeen different extracts were measured at concentrations (5-100µg ml-1) and the results were shown in (Figure 2). The extracts B8 & B10 showed significant free radical scavenging activity than other extracts of B7, B9 & B11.40 On the other hand extracts B9 & B7 showed higher antioxidant property at 100 and 50µg ml-1 concentrations but showed very lower activity at the concentration 10µg ml-1 (Figure 2).41 Vinson et al.42 also observed that red kidney beans had higher activity than other beans. Similarly, red grapes have higher antioxidant activity than green grapes, and red cabbage is higher than green cabbage.40 Among the extracts, (B9) butanol extracts of Memecylon showed the most scavenging by the lowest value of SC50 and a dose dependent manner in DPPH assay B7, B8, B9, B10 B12 B13 and B14 also showed the scavenging capacity among the crude extracts by the SC50 value (Table 4). In an experiment, Kumar et al.8 found some antioxidant activities in A. vasica, we also found anti-microbial activity but we did not measure the antioxidant activity of the leaves of that plant.
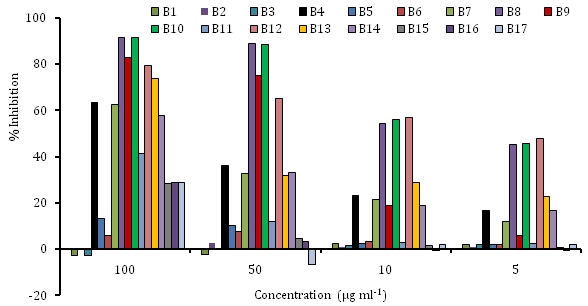
Figure 2 DPPH radical scavenging capacity of 17 medicinal plant extracts used in the experiments.
B1-n-Hexane extract of Pouzolzia, B2-EtOAc extract of Pouzolzia, B3- n-Hexane extract of Equisetum, B4-EtOAc extract of Equisetum, B5- n-BuOH extract of Equisetum, B6-Water extract of Equisetum; B7- n-hexane extract of Memecylon, B8-EtOAc extract of Memecylon, B9-n-BuOH extract of Memecylon, B10- MeOH extract of Memecylon, B11-water extract of Memecylon; B12-MeOH extract of Couroupita, B13- BuOH extract of Couroupita, B14-EtOAc extract of Couroupita; B15-PE extract of Datura, B16-n-hexane extract of Datura, B17-EtOAc extract of Datura.
Extracts |
Inhibition (%)a |
SC50 (µg ml-1)b |
Vit C |
98.106 |
4.1 |
B1 |
−c |
− |
B2 |
0.557 |
− |
B3 |
−c |
− |
B4 |
63.474 |
17.2 |
B5 |
13.252 |
− |
B6 |
5.902 |
− |
B7 |
62.568 |
7.6 |
B8 |
91.216 |
7.96 |
B9 |
82.675 |
5.1 |
B10 |
91.486 |
8.1 |
B11 |
41.313 |
− |
B12 |
79.269 |
7.93 |
B13 |
73.495 |
9.76 |
B14 |
57.799 |
14.56 |
B15 |
28.282 |
− |
B16 |
28.776 |
− |
B17 |
28.825 |
− |
Table 4 Free radical scavenging activity of the different crude extracts of medicinal plant sample
aPercent of inhibition and scavenging at 100µg ml-1 as a mean of triplicate experiments.
bSC 50 is defined as the concentration sufficient to obtain 50% of a maximum scavenging activity.
−c Negative value
B1-n-Hexane extract of Pouzolzia, B2-EtOAc extract of Pouzolzia, B3- n-Hexane extract of Equisetum, B4-EtOAc extract of Equisetum, B5- n-BuOH extract of Equisetum, B6-Water extract of Equisetum; B7- n-hexane extract of Memecylon, B8-EtOAc extract of Memecylon, B9-n-BuOH extract of Memecylon, B10- MeOH extract of Memecylon, B11-water extract of Memecylon; B12-MeOH extract of Couroupita, B13- BuOH extract of Couroupita, B14-EtOAc extract of Couroupita; B15-PE extract of Datura, B16-n-hex extract of Datura, B17-EtOAc extract of Datura
From the above result it can be concluded that different plant extract had shown significant antimicrobial and antioxidant properties. Among the samples, ethyl acetate extract of Aloe vera leaf gel showed scavenging and antimicrobial activity. Surprisingly petroleum ether extract of Datura seed showed remarkable microbial activity against the bacteria and viruses and it can be concluded that further investigation on those plants may contribute to the field of medicine and cosmetic industry.
None.
The author declares no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.