Advances in
eISSN: 2373-6402


Wheat and Barley is the most production and consumption grains in the world. The necrotrophic Fusarium spp is pathogen caused many diseases on plants, the major two disease caused by Fusarium on wheat is Fusarium Crown rot (FCR) and head blight (FHB), also known as scab. These both disease caused severe damage on yield quality and quantity, the produced seed seem to be small, shriveled pale white appearance with low contain of protein, that reflected on reduce yield up to 61%. Mycotoxins contamination in the grain, will lead to contamination the products that manufactured from these crops, as well as the contamination yield will be lower price than the healthy yield, which leads to a significant loss to the producer. The major FCR and FHB mycotoxins as DON, NIV, ZEN can caused many disease symptoms to consumers who consumes contaminate food, whether is human or animal, such as cancers, immunosuppression, fertilization problem, abortion and etc.
Keywords: triticum spp, hordeum vulgare, fusarium, mycotoxins
FCR, fusarium crown rot; FHB, fusarium head blight; DON, deoxynivalenol; ZON, zearalenone; AcDON, monoacetyl–deoxynivalenols (3–AcDON, 15–AcDON); BEA, beauvericin; DAS, diacetoxyscirpenol; ENS, enniatins; FUS, fusarenone–X (4–Acetyl–NIV); HT2, HT–2 toxin; MON, moniliformin; NEO, neosolaniol; NIV, nivalenol; T2, T–2 toxin; T2ol, T–2 tetraol; ZEN, zearalenone
Wheat and barley is the major crops cereal produce in the word. The global statistics indicate that wheat production in the world for 2014–2015 was 707.2million ton and for barley 135.65million ton, which form the proportion of one–third of the world’s total grain production.1,2
Fusarium crown rot (FCR)
Crown rot (FCR) caused by many kinds of Fusarium spp as F. pseudograminearum and F. culmorum is a common pathogen to FCR while F. grarnineurm group I, F. crookwellense, F. avenaceum and F. nivale.3–6 FCR disease infect the stem base of wheat and barley causing necrosis and dry rot of the crown bases always brown, often extending up 2–4nodes, basal stem and root tissue commonly known as crown rot.7 Whitehead formation is most severe in seasons with a wet start and dry climates, pinkish fungal growth may form on lower nodes especially during moist weather (Figure 1).

Figure 1 Different symptom and sing of Fusarium crown rot (FCR) on wheat.
(A,B) Pink/red fungal mycelium and discoloration of stem bases, Photo by SIMMYT.
(C) Whitehead symptom on spike right: non infected, left: infected (White head), Photo by Guihua Bai.
Fusarium Head Blight (Scab) (FHB)
FHB is caused by mainly fungus as F. graminearum group II (also known as Gibberella zeae sexual stage), F. culmorum, F. poae, F. avenaceum and F. culmorum.8,9 Other species, can caused the disease such as F. langsethiae, F. poae, F. sporotrichioides, and Microdochium nivale (formerly known as F. nivale).10,11 Disease symptoms are confined to the head, grain, and sometimes the peduncle (neck). Typically, the first noticeable symptom is bleaching of some or all of the spikelets while healthy heads are still green. As the fungus moves into the rachis, spikelets located above or below the initial infection point may also become bleached. In barley the symptom is little different, infection spikelest is located and don’t move in to rachis of spike. During humid and wet condition, pink to orange masses of spores may be visible on infected spikelets (Figure 2).12–14 Wet and warm weather during crop growing and maturation may favour to FHB,15 and hot and dry conditions increase CR disease.16
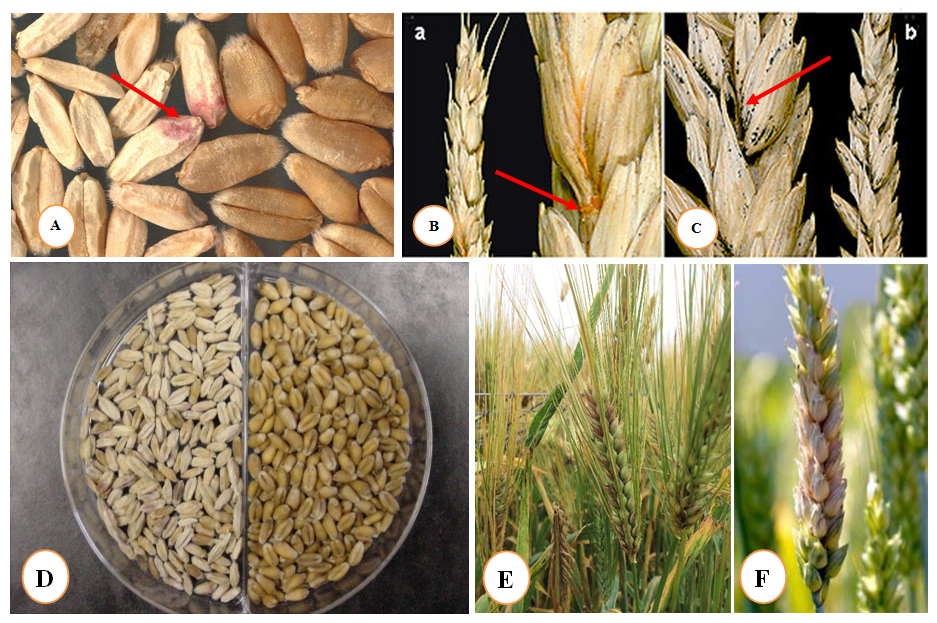
Figure 2 Head blight symptom on wheat and barley caused by Fusarium (FHB).
(A,B) Pink/red fungal mycelium and discoloration of stem bases, Photo by SIMMYT.
(C) Whitehead symptom on spike right: non infected, left: infected (White head), Photo by Guihua Bai.
Disease cycle
The causal agent for FCR and FHB is interplay. Infection occurs when spores land on susceptible wheat and barley heads. Heads are most susceptible at early flowering and infection may occur up to the soft dough stage, although severity is greatly reduced. If the flowers are infected just after their emergence kernels will not develop. Florets that are infected later will produce tombstones. Kernels that are infected by the pathogen during late kernel development may appear healthy, but be contaminated with mycotoxins. Fusarium crown and root rot can develop in the following season if infected seed is planted. Also maize is one of the crop that infected by same Fusarium spp that infected wheat and barly, so cultivation wheat or barly near or after maize rotation will increase inoculation which leaded to hight disease incidence (Figure 3).17
Yield losses
There are many diseases accompanying with wheat and barley cultivation, mostly about 9% of yield is losses by diseases in Kansas State was estimated for the last 20 year, Fusarium CR and HB constitutes about 0.29% losses from the total yield.18 Fusarium infection reduces quality and crop yield, both FHB and FCR become an endemic disease in wheat and barley grower’s area in the world wide.19 F. pseudograminearum is the predominant CR pathogen in the 11million/ha wheat growing region in Australia7 where, nearly $80 million each year is lost from reduced grain yield and quality due to CR.20 Both F. graminearum and F. pseudograminearum improved by bioassays that can caused equally severe CR disease.21
In the Pacific Northwest of the United States, F. pseudograminearum can reduce winter wheat yield by up to 61%.22 While in during 1998–2000 FHB inflicted an estimated US $ 2.7billion loss due to reduced yield and price discounts from lowered grain quality in the northern Great Plains and central USA.19 It has been reported that FCR could reduce wheat yield by up to 35% in the Pacific North– West of the United States.
Recent study found crown rot infestation causes an average of 25 percent yield loss in bread wheat and 58% loss in durum wheat across a wide range of environments in Australia, estimated annual yield loss of $23million Australian dollars per year in barley and in wheat and barley combined in Australia.20,23
Since 1993, North Dakota, South Dakota, and Minnesota have lost 73% of their malting barley market with losses in Minnesota alone approaching 95%, In barley, losses have been equally devastating with estimated losses from 1993 to 1999 totaling in excess of $400million. Yield losses in wheat since 1990 have exceeded 13Tg (500million bushels) with economic losses estimated at $2.5billion.24 FCR losses in yield arising on grain heads will be partially or half filled (Figure 2), or no grain in the head (preventing grain from forming in the heads of wheat) in severe infestation, as will reduction in grain quality and infected plants may also contaminated with mycotoxins.25
FHB can caused significant yield losses from floret sterility and light weight kernel are produced as a result of infection, Quality reduction may occurrence if the pathogen produce mycotoxins in seed face26,27 (Table 1).
Disease name |
%Percentage |
Rust disease |
4.33 |
Septoria complex |
0.84 |
Scab and crown rot |
0.29 |
Powdery medley |
0.09 |
Talk all |
0.03 |
Bunt & loose smut |
0.02 |
Table 1 Some statistical estimates for diseases losses on wheat yield, prepared by Kansas State University.18
Mycotoxin contamination and risk
The mycotoxin risks connected with the consumption of contaminated forage, grain and straw by livestock must not be ignored.28,29 High humidity and frequent rainfall increase Fusarium head blight (FHB) and crown rot disease in wheat and barley by dispersal and production spores from infected residue, the optimum temperatures for infection are between 75°F and 85°F.30 Mycotoxins are chemically and thermally stable contaminants which have toxic effects in animals and humans.31 They can accumulation in the grain and, when contaminated grain is consumed via feed or food products, cause a potential risk to human and animal health.32 Matny et al.,33 found that most samples of animal feed that collected from market and animal farm in Iraq was contaminated with DON toxin at 722µg/kg.
Toxigenic strains of F. culmorum have been divided into two types the main type B trichothecenes produced that is DON and NIV chemotypes, strains of DON–types also produced AcDON (3–AcDON),34–36 while strains of NIV–type were also able to produce FUS. In field trials, DON and NIV chemotypes exhibited different aggressiveness toward winter rye.35 FHB in wheat is widespread disease caused losses up to 25–50%, and high amounts of DON and its derivatives were frequently found in freshly harvested infected grains. In particular, a very high occurrence of DON incidence of 100%, range 7.25–36.25mg kg–1 was founding on wheat affected by FHB predominantly caused by F. graminearum, associated with consistent levels of 4,7–dideoxy–NIV (0.16–1.25mg kg–1 ).37 Most frequently encountered mycotoxins in field surveys in Europe infected wheat with FHB is deoxynivalenol (DON) and zearalenone (ZON) produced by F. graminearum and F. culmorum.29,38–40
In Poland, samples collected from wheat naturally infected with F. graminearum and F. culmorum were founded to be contaminated with DON and 3AcDON 100% and 80% respectively at very high concentrations up to 30.4 and 29.54mg kg–1 respectively, the levels of DON and 3AcDON in the chaff were 5–50times higher than in kernels41 (Table 2).
S. No |
Fungus Species |
Mycotoxins |
1 |
F. graminearum |
DON, NIV, ZEN, AcDON |
2 |
F. avenaceum |
MON, ENS, BEA |
3 |
F. culmorum |
DON, ZOH,NIV, ZEN |
4 |
F. poae |
NIV, FUS, BEA, DAS |
5 |
F. equiseti |
DAS, ZOH, ZEN |
6 |
F. tricinctum |
MON |
7 |
F. cerealis |
NIV, ZOH, FUS, ZEN |
8 |
F. sporotrichioides |
T2, NEO, HT2, T2ol |
9 |
F. acuminatum |
T2, NEO |
10 |
F. subglutinans |
MON |
Table 2 Some statistical estimates for diseases losses on wheat yield, prepared by Kansas State University.18
AcDON, monoacetyl-deoxynivalenols (3-AcDON, 15-AcDON); BEA, beauvericin; DAS, diacetoxyscirpenol; DON, deoxynivalenol (Vomitoxin); ENS, enniatins; FUS, fusarenone-X (4-Acetyl-NIV); HT2, HT-2 toxin; MON, moniliformin; NEO, neosolaniol; NIV, nivalenol; T2, T-2 toxin; T2ol, T-2 tetraol; ZEN, zearalenone; ZOH, zearalenols (α and β isomers).
The could place accompanied epidemics infection with F. sporotrichioides and F. poae may be lead to occurrence of T–2 derivatives (T2, HT2, T2ol), and DAS and MAS, respectively in infected grain, NIV and FUS toxin has also been founded to correlative to the activity of F. poae and F. cerealisin Sweden and other northern countries in grains from central to northeast Europe.42,43
MON was reported to be founded in freshly harvested durum wheat in Austria up to 0.88mg kg–1.44 In all these surveys, the MON contamination in kernels was well correlated with the F. avenaceum infection.45 Zearalenone is secondary metabolite produced by many Fusarium species, especially by F. graminearum and F. culmorum, this toxin has known as estrogenic properties, which means it can cause infertility, abortion, or other breeding problems. As little as 1 to 5ppm zearalenone in a feed ration may produce an estrogenic effect in swine.46 Study done by Matny47 about companion Fusarium pathogens to FCR and FHB to wheat, it’s found that F. verticillioides and F. proliferatum can produce with high amount of several kind of mycotoxin like DON, Fumonosin, ZEN and T2 toxin.
Economic impact of mycotoxins
Mycotoxins have significant economic impacts in many crops, especially in grain like wheat, maize, peanuts and other nut crops, cottonseed, and coffee. The Food and Agriculture Organization has estimated that 25% of the world’s crops are contaminated by mycotoxins each year, with annual losses of around 1billion metric tons of foods and food products. Economic losses occur because of:
Additional costs associated with mycotoxins include the cost of management at all levels prevention, sampling, and research costs. These economic impacts are felt all along the food and feed supply chains: crop producers, animal producers, grain handlers and distributors, processors, consumers, and society as a whole (due to health care impacts and productivity losses). Reduced crop value is a significant component of the losses caused by mycotoxins. This affects crops entered into local trade as well as crops intended for export.48
Fusarium head blight losses to barley producers in the upper Midwest of the USA over the years (1992–1997) have been estimated to as high as $364million. Deoxynivalenol reduces barley quality, lowering producer prices, and limiting the amount of uninfected barley available for the malting and brewing industries, which are not willing to accept barley with detectable levels of deoxynivalenol because conditions during malting promote Fusarium growth, leading to malted samples containing higher level of the toxin than in the original seeds. The limited amount of uninfected barley in the U.S. has had effects on the malting and brewing industry, which paid and extra $200million in 1994–1995 to get non–infected grain from Canada.49
Estimates of mycotoxins losses costs in the United States vary, one report estimated $0.5 to $1.5billion/year and another estimated $5billion/year for the U.S. and Canada. Neither of these estimates included human health impacts or crop yield losses. Losses associated with deoxynivalenol in the United States were estimated at $655 million/year, with the majority of the losses in wheat. In developing countries, few estimates are available.48 Human health impacts of mycotoxins are the most difficult to quantify. These effects are due to acute (single exposure) toxicoses and immunosuppression by mycotoxins, as well as chronic (repeated exposure) effects. Diseases modulated by mycotoxins accounted for 40% of lost disability–adjusted life years in a 1993 World Bank report on human health. Of the reported $900million impact of mycotoxin in Southeast Asia, $500million of the costs were related to human health effects.48
Trichothecenes toxins are the mean secondary metabolite produced by Fusarium spp, its very toxic to mammalian. Mode of action of these toxins is though inhibition of protein and DNA synthesis, impairment of ribosome function, inhibition of mitochondrial protein synthesis, induction of reparable single strand breaks in DNA and immunosuppression.50
Swine are very sensitive to Fusarium mycotoxins in their diet. Deoxynivalenol can cause reduced feed intake, and vomiting, reduced body weight gain or body loss. Deoxynivalenol has also been associated with abortions, stillbirths, and weak piglets. Immunosuppression with its associated increases in infection and disease incidence also increases production costs.51 Immune cells are more sensitive to DON than other cell types due to the induction of a T–cell activation response by increased intracellular calcium levels.52
Zearalenone affects reproduction in swine and can have major economic effects in number of pigs produced per sow per year determine profit margins. Based on 1991 prices, it was calculated that a 10 or 20% reduction in farrowing rate combined with a 10 or 20% reduction in growth (as may occur if deoxynivalenol and zearalenone contaminated feed was consumed) would result in a 17 to 44% reduction in profit margins, due to increased feeding and veterinary costs per head, and a decline in the number of pigs marketed.53
This study highlights on the economic loses consequent form disease damage to plant and reflected on yield, and health risk on consumers whether is human or animal, The fact that the pathogen Fusarium spp is responsible for producing mycotoxins in the infected cereal and the straw that produced from this plants. Therefore, preventive measures should be taken to reduce pathogen infection and spread, by using Integrate pest management for the disease through using resistance cultivars, biological control, rotation, crop management etc.
None.
The author declares no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.