|
CD166 as a stem cell marker? a potential target for therapy colorectal cancer?
1Department of Biology, Islamic Azad University, Iran
2Cancer Institute, University of Medical Sciences, Iran 3Department of chemistry, University of Sistan and Baluchestan, Iran Received: November 13, 2016 | Published: November 23, 2016
Correspondence:
Hassan Dana, Department of Biology, Islamic Azad University, Damghan Branch, Damghan, Iran, Tel 0098 91050 40602, Email
Citation:
Dana H, Marmari V, Mahmoodi G, et al. CD166 as a stem cell marker? a potential target for therapy colorectal cancer? J Stem Cell Res Ther. 2016;1(6):226‒229. DOI:
10.15406/jsrt.2016.01.00041
AbstratCD166 (Activated leukocyte cell adhesion molecule (ALCAM)) is a member of the immunoglobulin superfamily, which expressed by various cells in several tissues including colorectal cancer (CRC). Many studies have reported the prognostic predictive value of CD166 as a cancer stem cell marker in CRC. CD166 gained increasing attention regarding tumor progression and metastatic spread in CRC. CD166 potentially represents either diagnostic or therapeutic capacities for CRC. This Mini review aimed to clarify the role and significance of CD166 expression in CRC. Keywords: cd166, alcam, stem cell, CRC, immunoglobulin, cancer stem Cell AbbreviationsCSC, cancer stem cell; ALCAM, activated leukocyte cell adhesion molecule; CRC, colorectal cancer IntroductionColorectal cancer (CRC) is the third most common cancer in men and the second in women worldwide, accounting for almost 10% of all cases.1,2 The USA and Europe have high rates compared to Africa and Asia.3,4 Only in the United States, nearly 135,000 new cases diagnosed annually that about 50,000 people will die of the disease.5 In Iran CRC is the third most common cancer in women and the Fifth in men.6 Two big problem is the high ability of CRC to form secondary tumours, particularly in the lung and liver and the high heterogeneity of the genetic and epigenetic changes among the individual tumours. Continual development of CRC is described on the Figure 1.7 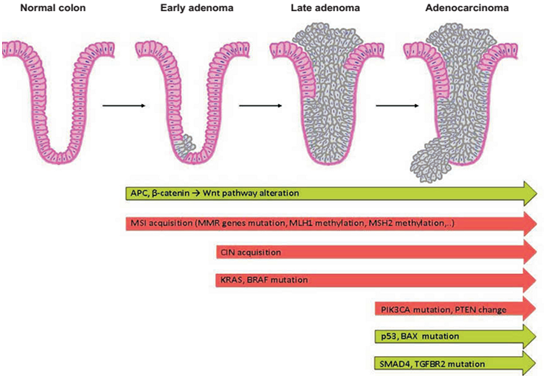
Figure 1 Cells of the colon crypt accumulate mutations and start to proliferate. In the Green arrows you can see inactivation of antioncogenes, in the red arrows are mentioned most important changes in oncogenes.7
Activated leukocyte cell adhesion molecule (ALCAM) also known as CD166, which functions as a cell–cell adhesion molecule in homophilic (ALCAM-ALCAM) and heterophilic (ALCAM-CD6) interactions between adjacent cells in different tissues.8–13 It is detectable in a wide variety of cell types, including lymphoid bone marrow, cells, hepatocytes, fibroblasts, neuronal cells, myeloid cells, and epithelial cells.14 CD166, which was first described as a CD6 ligand on leukocytes, is a highly conserved 110-kDa multi domain transmembrane type-1 glycoprotein of the immunoglobulin super family.15,16 It has a variety of functions in normal tissues, such as migration of monocytes across endothelia, intravasation of leukocytes into the central nervous system and T-cell activation.17 CD166 has been cloned in multiple species and has different names depending on the species and laboratory that cloned it: human melanoma metastasis clone D (MEMD), chicken neural adhesion molecule BEN/SC-1/DM GRASP, mouse/human ALCAM (CD166), rat KG-CAM, HB2 and fish neurolin.18,19 CD166 is, composed of 16 exons and has a size of over 200kb, with an extracellular domain of 500 amino acids, a transmembrane domain of 22 amino acids and a short cytoplasmic domain of 34 amino acids. It’s consists of five extracellular Ig domains (2 NH2-terminal, membrane-distal variable-(V)-type and 3 membrane-proximal constant-(C2)-type Ig folds) and the human gene for it is located on chromosome 3 (3q13.1q13.2)20 (Figure 2). 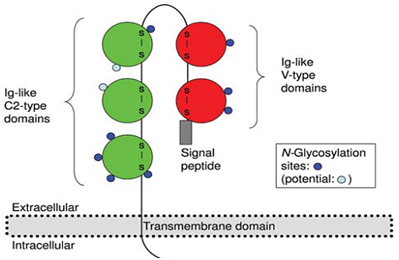
Figure 2 Topology of ALCAM. The two Ig-like V-type domains and the three Ig-like C2-type domains are shown in red and green; glycosylation sites and the cytoplasmic domain are indicated.20
Adhesion molecules are divided into main categories, which include cadherins, integrins, mucins, selectins and immunoglobulins. Adhesion molecules can be involved in tumor cell-endothelial cell adhesion, tumor cell-matrix adhesion or tumor cell-tumor cell adhesion; all of these adhesions are necessary at different times during metastasis or primary tumor formation.19 The distribution of CD166 in specific cell and tissue offer their involvement in the maintenance and development of tissue architecture, in hematopoiesis, in neurogenesis, immune responses and in tumor progression.21 Malfunction, overexpression, or loss of CD166 may contribute to the detachment of tumor cells and therefore to local invasion and tumor progression.22 CD166 expression is pathologically correlated with aggressive disease in a variety of cancers including breast,23 esophageal,24 prostate,25 melanoma,26 ovarian, bladder and colorectal.27 DiscussionCD166 is a marker of CRC stem cells that it has potential as a therapeutic target for CRC.27,28 CD166 is important for cell survival and motility, cell growth, and also for invasion during metastases and tumor progression. Overexpression, loss, or malfunction of CD166 may contribute to the detachment of tumor cells and therefore to local invasion and tumor progression.22 Tachezy et al.12 evaluated the expression of CD166 in CRC. This study was performed to retrospectively evaluate the expression of CD166 in CRC tissues of primary and metastatic sites and to determine whether ALCAM could serve as a diagnostic and prognostic marker.12 Expression of CD166 in CRC has been analyzed by several groups with conflicting results.29 In CRC a pathologically altered CD166 expression has been observed and could be associated with tumorgenesis and tumor progression.22 In human CRC, aberrant cell surface CD166 expression is strongly correlated with a 15-month shortened survival.27 In a research the expression of CD166 in CRC with using immunohistochemical staining with a semiquantitative scoring system, cytoplasmic and membranous immunoreactivity were analyzed. In research the expression of CD166 in CRC with using immunohistochemical staining with a semiquantitative scoring system, cytoplasmic and membranous immunoreactivity were analyzed. 58.6% had strong cytoplasmic staining and 30.6% had strong membranous staining (compared with normal epithelium) of the 111 CRC studied. Membranous CD166 expression correlated significantly with shortened patient survival. The authors proposed that upregulation of CD166 is an early event in the malignant transformation in CRC because it was identified in cancers, which are considered to be precursor lesions.19 CD166 shedding in CRC, defined as detection of the intracellular domain in the absence of the corresponding extracellular domain, was significantly elevated in patients with CRC and correlated with reduced survival. Conversely, retention of intact CD166 was associated with improved survival, thereby confirming that CD166 shedding is associated with poor patient outcome.30 ConclusionCD166 is considered as a stem cell marker in colorectal cancer. Stem cells are determined as cells with the capability to perpetuate themselves through self-renewal and to produce mature cells of a special tissue through differentiation. As with other membrane proteins, CD166 represents a potential target for therapy, and its utility as a drug target may be further enhanced by ligand-induced endocytosis. Concentrate on cancer initiation and progression has dominated the endeavor to better guide therapeutic approaches and understand disease pathology. As such, Cancer stem cell (CSC) hypothesis, which suggests that cancer is driven by cells harboring stem cell-like qualities, offers one explanation for why many current therapeutic approaches ultimately result in relapse of disease. Today the techniques that are commonly used in chemical therapy for the treatment of cancer, only target the differentiated or differentiating cells which form a major part of the tumor mass. The fact, that these cells only form the tumor volume but they cannot generate new cell and do not play a role in the disease progression and the development of tumor, is not noticed. In this hypothesis, some CSCs or cancer-initiating cells may be quiescent and, thus, evade eradication by standard cytotoxic therapies designed to target proliferating cells. These surviving cells can then proceed to support tumor growth and have the potential to initiate recurrent or metastatic disease.27 The focus on these facts, and as CD166 is assumed as stem cells marker and is expressed highly in colorectal cancer, it can be an appropriate remedial potential to treat them. CD166 is a cell-surface antigen that is proposed as the antigen of cancer stem cell in CRC. Active immunotherapy encompasses a diverse range of strategies, some of which target multiple, undefined antigens whereas others specifically target a particular antigen or a group of antigens. Recently, the recognition of the tumor related antigen has introduced a new foundation in the immunotherapy of special antigens. The major aim of the vaccine experiments initiated from the last decade was to induct the specific immune response against the cancer antigens.31,32 CD166 expression is a positive prognostic marker for overall survival of CRC patients, and its detection might help to optimize the existing prognostic staging system. Elevated expression in higher differentiated tumors might indicate a potential role in the early steps of tumorigenesis, and its loss might be associated with reduced cellular adhesion, resulting in a higher metastatic potential of the tumor.12,33 AcknowledgementsNone. Conflict of interestThe author declares no conflict of interest. References
 ©2016 Dana et al. This is an open access article distributed under the terms of the Creative Commons Attribution License
, which
permits unrestricted use, distribution, and build upon your work non-commercially.
©2016 Dana et al. This is an open access article distributed under the terms of the Creative Commons Attribution License
, which
permits unrestricted use, distribution, and build upon your work non-commercially.
|