Journal of
eISSN: 2377-4282


Review Article Volume 1 Issue 1
Institute of Science and Technology in Medicine, Keele University, UK
Correspondence: Clare Hoskins, Institute of Science and Technology in Medicine, Keele University, Keele, Staffordshire, UK, Tel 441782-734799
Received: September 13, 2014 | Published: October 1, 2014
Citation: Malekigorji M, Curtis ADM, Hoskins C (2014) The Use of Iron Oxide Nanoparticles for Pancreatic Cancer Therapy. J Nanomed Res 1(1): 00004. DOI: 10.15406/jnmr.2014.01.00004
Over the last decade major advances have been made in the treatment of cancer such as breast and leukaemia. However, no satisfactory progress has been made in the effective treatment of pancreatic cancer. Treatment of this disease is hindered by resistance of tumor cells to chemotherapy and impaired drug delivery after administration. Pancreatic adenocarcinomas characteristically form a dense stroma, which hinders drug penetration. Increasing administration dosage may provide increased therapeutic effects. However, toxic drug molecules do not act selectively to tumor cells and, as such; a vast range of undesirable side effects can be experienced. Nano-sized formulations of cytotoxic agents have proved to passively target pancreatic adenocarcinomas and promote increased drug efficacy. This is thought to be due to the accumulation via enhanced permeability and retention resulting in deeper drug penetration. Nanoparticles with easily modified surfaces have been investigated extensively in recent years and play a pivotal role in biomedicine. In recent years, magnetic NPs have been increasingly explored for clinical applications, such as drug delivery, magnetic resonance imaging and magnetic fluid hyperthermia for diagnosis and cancer therapy. In comparison with traditional cancer therapy, magnetic field operated therapeutic approaches can treat cancer in an unconventional but more effective and safer way. In this literature review, we highlight the recent advances in the use of iron oxide nanoparticles in pancreatic cancer therapy.
Keywords: Metallic iron oxide, Drug delivery, Pancreatic cancer, Magnetic resonance imaging, Theranostic
MNPs, Magnetic Iron Oxide Nanoparticles; EPR, Enhanced Permeability and Retention; MRI, Magnetic Resonance Imaging; NPs, Nanoparticles; SPION, Super Paramagnetic Iron Oxide Nanoparticles; PDAC, Pancreatic Ductal Adenocarcinoma; RES, Reticulo Endothelial System
Cancer is described as the most hazardous class of disease categorized by uncontrolled cell growth.1,2 It is the third leading cause of death (after heart disease and stroke) in developed countries as well as the second leading cause of death (after heart disease) in the United States. Studies have demonstrated that there were 10 million new instances, about 6 million deaths and 22 million patients living with cancer worldwide in the year 2000.3
Pancreatic ductal adenocarcinoma, known as PDAC, is the most common epithelial, exocrine pancreatic malignancy, i.e. showing for more than 80% of the malignant neoplasm of the pancreas.4 It is still the fourth most common cause of cancer-related death in the Western world.5 There is a direct correlation between pancreatic cancer diagnosis and increasing age with a peak incidence of the disease occurring in the 65–75 year age group.6 Patients with untreated metastatic pancreatic cancer normally live for 3–5 months and 6–10 months for locally advanced disease.7 The majority of cases are diagnosed in the malignant stages, making curative therapy impossible and leading to poor prognosis and incidence equalling mortality.8 In the year 2000, there were 217,000 new cases of pancreatic cancer with 213,000 deaths worldwide, while in Europe there were 60,139 new patients with 64,801 deaths.9 In the UK, 7,152 new cases were seen with 7,250 deaths as a result of PDAC.10 PDAC affects more western/industrialized citizens than other parts of the world. The highest prevalence has been shown among Maoris in native Hawaiians, New Zealand, and Black American societies, while people living in India and Nigeria have the lowest reported morbidity.11,12
Gemcitabine is the only chemotherapy available clinically for pancreatic cancer treatment. Unfortunately, it proves effective in as few as 23.8% of patients with the only alternative being surgical removal of the localized tumor (Figure 1).13-15 Thus, there is huge requirement to increase the efficacy of this treatment as well as exploring alternative therapies.
Nanoscience and nanotechnology has become a versatile and promising platform for creating novel materials with enhanced properties and potential applications in cancer therapy. Nanotechnology, a wide research field which includes chemistry, engineering, biology and medicine, has excellent potential for early detection, accurate diagnosis, and treatment of cancer.16 Using materials at nanoscale leads to a “big revolution” in healthcare and medical therapies. Nanoparticles (NPs) are usually smaller than several hundred nanometres: in comparison with large bio molecules such as antibodies, receptors and enzymes. NPs can undergo many interactions with biological molecules both on the surface of and inside the cells due to their size (one hundred to ten thousand times smaller than human cells), which may revolutionize cancer diagnosis and treatment. The most commonly studied NPs include quantum dots.17 carbon nanotubes .18 paramagnetic NPs.19 liposomes.20 gold NPs.21 polymeric, lipid and silver NPs.22,23 Quantum dots are useful in biological labelling and detection due to their size-dependent fluorescence properties.24-26 Magnetic NPs have been used for cell sorting.27 MRI.28-30 drug delivery.31-33 and magnetic hyperthermia therapy.34-36 Lipid and polymeric NPs have been used to encapsulate therapeutic molecules to increase drug solubility, safety and delivery efficiency based on the enhanced permeability and retention (EPR) effect of the tumor tissue.37,38 Carbon-based NPs, especially carbon nanotubes, have found increasing interest from the point-of-view of biomedical applications such as photo thermal therapy.39 and drug delivery.40-42
The suspension stability (i.e. ability to remain in solution indefinitely) of NPs in physiological conditions is one of the most vital factors for their successful usage in biomedicine. This is dependent on the physical and chemical composition of the NPs, the charge in particular. When discussing NPs, size and colloidal stability (i.e. the capability of particles to resist aggregation, particularly for magnetic NPs) in physiological environments is a crucial factor to show whether a particle has the potential for clinical application. Although characterization of a particle’s properties such as surface charge, surface area and crystallinity have been successively taken into account.43 an in depth understanding of the basic mechanisms including NP-protein interactions and their colloidal behaviour in different physiological environments is needed.44 The therapeutic outcome of chemotherapeutic treatments has considerably improved in the last decade but one of the main reasons for failure is correlated with the presence of multidrug resistance-associated protein in cancer cells. Once in biological media, proteins and other biological molecules quickly compete to bind onto the NP surface, leading to the formation of a protein corona (Figure 2).
This critically defines the biological identity of the particle, whilst shielding its original surface properties. The biophysical properties of such a particle-protein complex may differ significantly from those of the formulated particle. Therefore, the biological activity and particle bio distribution are predominantly influenced by the nanoparticle-protein complexes.
Nano-sized formulations of cytotoxic agents have proved to passively target pancreatic adenocarcinomas and promote increased drug efficacy. This is thought to be due to the accumulation via EPR resulting in deeper drug penetration (Figure 3). The blood vessels supplying the blood to cancerous tissue are leaky and disorganized. The tumor tissues also experience poor lymphatic drainage. Nanoparticle size plays a significant role in their ability to extravasate from the bloodstream and reach the tumor tissue. The size of NPs used in a drug delivery system should be large enough to prevent filtration by the kidney and small enough to avoid the capture by the liver and spleen.45 Nano-structures (<120nm) are capable of entering the highly permeable blood capillaries which supply the rapidly growing tumors.46 This however does not occur in normal tissue as the blood vessels are well formed and non-porous. Once inside the capillaries, they accumulate and are retained in the tumor as a result of the poor lymphatic drainage. Additionally, formulation with appropriate nanoparticles can enhance physicochemical properties such as aqueous solubility leading to higher therapeutic efficiency.
Previously, the use of nano-sized graft amphiphilic polymers (Poly(allylamine)g-cholesterol) for encapsulation and delivery of a novel drug for pancreatic cancer (Bisnaphthalimidopropyldiaaminooctane, BNIPDaoct) was reported.47 BNIPs are a series of novel compounds which have exhibited exciting potential as chemotherapy agents.47-49 However their clinical use is hindered by undesirable physical properties; hence they require formulation before administration. Here the polymer nano-aggregates formed were passively targeted. The formulation was administered in vivo on a pancreatic cancer model using nude tumor bearing mice over a period of 4 wks. Although the BNIPDaoct dose was eight-fold less than clinically used gemcitabine, the formulation was able to reduce tumor growth in xenograft mice with comparable results.47 This study highlights the undoubtedly promising potential of nanotechnology formulations in the delivery of drug molecules which are otherwise discarded due to their poor physicochemical properties.
However, some studies have suggested that the EPR effect alone is not sufficient for treatment of diseases such as pancreatic cancer.50-52 This is due to poor diffusion of nanoparticles within the dense collagen matrix of the interstitial spaces and hence poor tumor penetration can occur. To overcome such issues, multistage nanoparticles which possess the ability to change size in different media have been developed. Wong et al.52 developed gelatin coated quantum dots which undergo structural degradation from 100 nm to 10 nm upon exposure to the tumor microenvironment. This proof of principle study showed how deeper tissue penetration could be achieved with the smaller nanoparticles compared with the initial 100 nm particles. Future studies are now concerned with incorporating nanomedicine carriers within the system in order to allow for deep penetration chemotherapy.
Over the past decade metallic NPs have been extensively studied for their potential in biomedical applications.53-55 Compared with other NPs, metallic NPs have proven to have unique chemical and physical properties based on their quantum-size which lead to a range of interesting biomedical applications. They can be easily synthesized with a high level of control of their size, shape and composition. Commonly studied metallic NPs include metal oxides such as iron oxide.56 copper oxide.57 zinc oxide.58 and aluminium oxide.59 It is essential that metallic NPs are carefully surface engineered before introduction into biological environments due to their inherent instability and associated toxicity.60-62 The particular focus of this review is on the use of iron oxide nanostructures for pancreatic cancer.
Iron oxide metallic nanoparticles
Magnetic iron oxide nanoparticles (MNPs) have been the focus of vast scientific interest due to their potential for numerous applications in nanomedicine (Figure 4).63,64 These include being utilized in the recovery of metal ions and dyes, magnetic bio separation, targeted therapy, drug delivery, biological detection and imaging. Magnetic separation techniques possess the advantage of rapid, high efficacy, and cost-effectiveness. Also, they have been shown to be highly efficient as supports in heterogeneous catalytic reactions owing to the high specific area and magnetic recoverability. MNPs possess large surface area to volume ratios due to their nano-size, low surface charge at physiological pH and they aggregate easily in solution due to their inherent magnetic nature. In some cases an unwanted aggregation may decrease the long term stability of products leading to large nanoparticle clusters which are undesirable for medical application. Additionally, degradation of iron oxide into free ions in physiological environments.65 has been reported to increase free radical production in cells causing damage which may cause cell death.66-68 Therefore, these particles are commonly coated with organic macromolecules such as poly(acrylic acid) PAA.69 Dextran.70 and poly(ethyleneimine) (PEI).71 or coatings such as silica.72 carbon.73 or precious metals (e.g. gold or silver).74 Common problems with chemically synthesized hybrid structures are the multiplicity of synthesis steps, incomplete understanding of the fundamentals of particle formation and, finally, a broad range of structures with ill-controlled configurations are produced.75,76
The most desirable MNPs are composed of the iron oxide core surrounded by a biocompatible surface coating which shows stabilization under physiological environment. With further surface modification, the attachment of functional ligands and drug molecules is possible allowing for increased functionality. The modification and functionalization of MNPs have been shown to improve their magnetic properties and influence their behaviour in vivo.56,77 MNPs have been clinically exploited as contrast enhancement agents in magnetic resonance imaging (MRI). This is due to their ability to enhance the proton relaxation of specific tissue.
Types of iron oxide nanoparticles
Super paramagnetic iron oxide nanoparticles: Iron oxide nanoparticles are typically classed as super paramagnetic or ferromagnetic based on their size. Although the majority of studies in nanomedicine are based on super paramagnetic NPs, some studies have also highlighted the potential of ferromagnetic.
The term superparamagnetism is assigned to the magnetic phenomena observed in fine magnetic particle systems exhibiting close similarities to atomic paramagnetism. Freeman et al. were the first to describe the concept of the use of magnetism in medicine in the 1970s.31 Superparamagnetism occurs when the size of the nanoparticulate is so small (20 nm or less) resulting in a single-domain nanoparticle whose magnetic anisotropy barrier is lower than the thermal energy, and in consequence, the orientation of the magnetic moment of the particle is unstable due to the thermal agitation, like the atomic moments in a paramagnet. Unlike their paramagnetic counterparts, super paramagnetic materials retain no remnant magnetization upon the removal of an external magnetic field.78,79 Super paramagnetic iron oxide nanoparticles (SPIONs) are small synthetic α-Fe2O3 (hematite), γ-Fe2O3 (maghemite) or Fe3O4 (magnetite) particles with a core diameter ranging from 10 nm to 20 nm. SPIONs have been exploited as targeted magnetic resonance contrast agents for MRI, which improve diagnosis of progressive diseases like cancer in their early phases.80 From a drug delivery viewpoint, targeting of cancer is the most pursued area, with emphasis on delivery of radio therapeutics and chemotherapeutics.81 However, increasing applications of SPIONs have also been used in the areas of cell death by using local hyperthermia, gene delivery, and delivery of antibodies and peptides to their site of action.
Different synthetic routes have been established in the fabrication of SPIONS ranging from physical methods such as mechanical grinding and bio mineralization processes to chemical means such as co precipitation methods, micro emulsion methods, hydrothermal syntheses, sol-gel syntheses, electrochemical methods, sonochemical reactions, polyol methods, flame-assisted methods, thermal decomposition methods, etc. By using co precipitation methods the size of the particle can be controlled by three different factors: temperature, base concentration and presence of surfactants.82 In comparison with the physical methods and the bio mineralization processes, the chemical methods, especially the solution-based synthetic ways, are generally more suitable for producing SPIONs for MRI application because they show great advantages in controlling the particle size, size distribution, degree of crystallinity and phase purity, which are the most fundamental parameters in terms of MRI applications.
There are two main pathways, through which SPIONs may be prematurely removed from circulation, either via uptake by the RES (200 nm) or through renal clearance mechanism (Ps<5.5 nm). Particles between 10 and 100 nm in diameter have the greatest circulation time. Their reduced surface area, in comparison with large SPIONs decreases the space accessible for adsorption of RES proteins. Moreover, they are still large enough to escape renal clearance. The chemical nature of the surface itself is also an important factor when designing stealthy NPs. For example some evidence suggest that the RES interaction of SPIONs <40 nm in diameter tend to be influenced more by their surface properties than their size. A negatively charged surface increases the attachment of plasma proteins which leads to enhanced uptake whereas a positively charged surface can cause the SPIONs to adhere to cells in a nonspecific manner. In general, hydrophilic and neutral surface are preferred to minimize opsonisation and clearance.83
Two of the NPs are commercially available (amionSPARK® and Feraheme®), but have been chemically modified to achieve a specific function.84 This coating allows for addition of functional ligands, such as radioactive ions for PET imaging and fluorophores for fluorescent imaging.
Ferromagnetic iron oxide nanoparticles: Ferromagnetic iron oxide NPs offer some attractive possibilities in biomedicine. First of all, they have controllable sizes ranging from a few nanometres up to tens of nanometres, which locates them at dimensions that are smaller than or comparable to those of a virus (20-450 nm), a cell (10-100 µm), a protein (5-50 nm) or a gene (2 nm wide and 10-100 nm long). This demonstrates that they may enter to a biological entity of interest. These nanomaterials can be coated with bio molecules for binding to or interacting with a biological entity, in order to produce a controllable addressing or tagging tool.85 Moreover, these magnetic NPs can be influenced by external magnetic fields. In electromagnetic fields, energy can be transferred from the exciting fields to the nanoparticle, thus, ferromagnetic iron oxide NPs can be designed to resonantly react to a time-changing magnetic fields. Ferromagnetic nanoparticles possess stronger magnetic potential than their SPION counterparts which is attractive for external magnetic guidance and increased contrast-ability.86-88 These are made available in biomedicine due to the special physical properties of ferromagnetic iron oxide NPs. Commonly ferromagnetic iron oxide NPs are synthesised via co precipitation; like SPIONS size, shape and crystallinity can be tailored based on reaction conditions. While SPIONs are less magnetic they are often preferred due to the relative instability of ferromagnetic, making them difficult to suspend due to aggregation and making them more challenging for biomedical applications.
Iron oxide for MRI
Iron oxide NPs was used for the first time in clinic to image liver tumor and metastases. Multi-functional MNPs are currently under evaluation for use in improving the delineation of brain tumor boundaries and quantifying tumor volumes .89,90 Many SPIONs are in the first stage of clinical trials or experimental study.91-95 and several formulations have been approved for clinical use for medical imaging and therapeutic applications. Some examples are: Lumiren® for bowel imaging.96 Feridex IV® as a liver and spleen imaging agent.97 Combidex® for lymph node metastases imaging.98 and most recently, Ferumoxytol® for iron replacement therapy.99 Despite the great success of modern nanomedicine in creating this new class of medications, there is still a lack of complete theoretical models describing the electron structure of the complex compounds of iron oxide NPs with medical molecules.
MRI is a well-suited tool for in vivo cell tracking because of its high spatial resolution. It is due to the fact that hydrogen protons will align and process through which these protons coming back to their initial state is known as the relaxation phenomenon. Two independent processes, longitude relaxation (T1 recovery) and transverse relaxation (T2 decay) are monitored to generate the MR image. Paramagnetic and super paramagnetic contrast agents change the relaxation time significantly by transferring their magnetic relaxation to the surrounding nuclei, so by selecting a specific monitoring delay the signal between contrasted and non-contrasted areas becomes significant (Figure 5). In recent years, the use of SPIONs to visualize cell migration has been used clinically, showing the potential capabilities of monitoring cellular therapies with MRI. The detection threshold for SPION-labelled cells is influenced by acquisition parameters and magnetic field strength. The drawback of MRI is that it requires high concentrations of contrast agents because it has low sensitivity. A high concentration of iron oxide increases toxicity and causes concern. However, making aggregated particles is an alternative way to increase the sensitivity. Aggregated particles have a stronger magnetic field, therefore increasing the concentration is not necessary.53 In line with the recent studies on cellular fate and toxicity of polymer coated iron oxides, most of the iron oxide nanoparticulate which were used clinically as contrast agents has been removed from use in humans in the UK. These include Feridex IV® and Luminex®. As such research is now underway to use highly rigid coatings which produce biocompatible nanoparticulate which maintain the unique contrast ability of their predecessors.
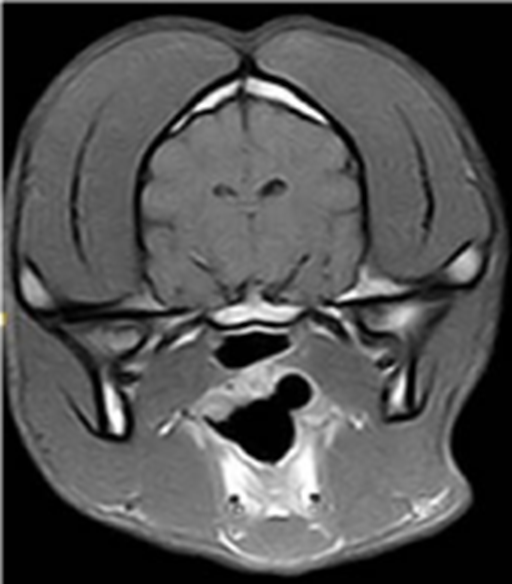
Figure 5 Typical MR image of a healthy canine brain using T2 imaging agent showing difference in contrast between various areas.
Wu and colleagues investigated MR imaging of human pancreatic cancer xenograft labelled with SPIONs in nude mice.100 Tumor xenografts were induced in nude mice by the inoculation of human pancreatic cancer cells labelled with SPIONs. The unlabelled cancer cells served as a control. MR imaging was performed with a 1.5 T MR scanner for the tumor xenograft at the first, second and third week after the inoculation. Wu found that the tumor xenograft was induced in 100% nude mice on MR imaging for both groups in the first week after the inoculation. In the SPION group, the tumors showed homogeneous hypo intensity on T1 - and T2 -weighted and FIESTA images 1 week after inoculation. Two and 3 weeks after inoculation, the centre of the tumors was still hypo intense on all the above sequences. The tumor periphery is intense on T1 -weighted, and hyper intense on T2 -weighted and FIESTA images. The tumors in the control group were homogeneously hypo intense or is intense on T1 -weighted, and hyper intense on T2 -weighted and FIESTA images in the first, second and third week after the inoculation. The size and signal-to-noise ratio of the tumor centre in the group inoculated with the SPIONs decreased in all T1 - and T2 -weighted images and FIESTA. These results highlight that human pancreatic cancer cells labelled with SPIONs can induce tumor xenograft in nude mice and MRI can monitor the kinetics of SPIO distribution in tumor xenografts.100
Iron oxide for magnetic hyperthermia
Magnetic hyperthermia is renowned as an alternative treatment which could be used alone or as an adjunct to chemotherapy and/or radiation for cancer treatment. Iron oxide NPs will generate heat when subjected to strong magnetic fields. Upon application of an electromagnetic field, the moments of the iron oxide NPs fully or partially align in the direction of the field. At a unique frequency to the NPs, the magnetic moments lag behind the field leading to a lag in the magnetic response. As such, the magnetic moments do not follow the same trajectory during the reversal of orientation of the applied magnetic field. A hysteresis loop is generated which is evident when the reversal is inhibited the associated energy loss is dissipated in the form of heat energy. In single domain materials, magnetic moment can fluctuate randomly by thermal fluctuation at high temperatures. At lower temperatures, the thermal energy is reduced resulting in a blocking of the magnetic moments. This is known as the blocking temperature and is specific to each size and shape of NP. Smaller particles occupy smaller volumes and hence possess a lower energy barrier and lower blocking temperature.
Magnetic crystal suspensions of iron oxide NP’s store the energy of alternating magnetic fields and release this energy as heat causing hyperthermic stress in cancer cells.101 In targeted magnetic hyperthermia treatment of cancers, MNPs act as thermal seeds under an alternating magnetic field (Figure 6).86-88 Increasing temperature above 40°C improves the radiation effect and causes thermoablation of the cancer cells. In one interesting study Kossatz and co-workers investigated the therapeutic effects of magnetic hyperthermia induced by super paramagnetic iron oxide nanoparticles on pancreatic cancer (BxPC-3) xenografts in mice in vivo. The SPIONs were injected intratumorally into the pancreatic model followed by cumulative heating to 43˚C. Histological analysis of magnetic hyperthermia treated tumor tissue exhibited alterations in cell viability (apoptosis and necrosis) and showed a decreased cell proliferation compared with the controls. This highlights the use of magnetic hyperthermia in pancreatic cancer therapy.102
In another study Basel loaded MNPs into mouse monocyte-macrophage like (RAW264.7) cells. These cells are renowned for their tumor homing activity. A murine xenograft model of disseminated peritoneal pancreatic cancer was then generated by intraperitoneal injection of Pan-02 cells. After tumor development, monocyte/macrophage-like cells loaded with MNPs were injected intraperitoneally and allowed to migrate into the tumor. After three days the mice were placed in an alternating magnetic field for 20 min to initiate hyperthermia. This treatment regimen was repeated three times. A survival study demonstrated that this technology significantly increased survival in this murine pancreatic cancer model. The average post-tumor insertion life expectancy increased to 31%. It was concluded that this system has great potential to become a useful method for specifically and actively delivering nanoparticles for local hyperthermia treatment of cancer.103
One drawback to the current state of this technology is the inconsistencies between experimental results and predictions of the amount of heat generated by the MNPs based on the existing simple models, and this has been a major disadvantage to optimize the design of magnetic particles for practical application.104
Iron oxide nanoparticles as vehicles for chemotherapy
Iron oxide NPs are increasingly being investigated for their potential as drug delivery vehicles for chemotherapy.105-107 Nano-sized formulations of cytotoxic agents have proved to passively target pancreatic adenocarcinomas and promote increased drug efficacy.47 This is thought to be due to the accumulation via EPR resulting in deeper drug penetration. This factor combined with the rapid diagnostic and treatment platform of this technology results in a system with great potential to act as a localised therapy with reduced dosages, thus minimising harsh side effects and improve clinical outcomes for patients with pancreatic cancer.
Many chemical methods have been applied for the conjugation of therapeutic, targeting and imaging carrier molecules with NP surfaces. These can be classified into covalent linkage strategies (direct nanoparticle conjugation, covalent linker chemistry, click chemistry) and physical interactions (hydrophilic/hydrophobic, electrostatic and affinity interactions). Physicochemical properties, functional groups found on the NPs coating and ligands are factors which influence the choice of chemistry. The primitive aim is to bind the targeting, therapeutic or imaging moiety without compromising its functionality. Functionality in such assemblies is dictated by the nature of the ligand (e.g. conformation of bio molecules) and the manner in which it is attached. For instance, if an antibody is conjugated to the NP but its recognition site is shielded by proteins, it might lose its potency to bind or reach to a target.
The surface of iron oxide NPs has been modified with anticancer drugs such as doxorubicin (Dox).108 Catechin–Dextran.109 and Paclitaxel.108 Catechin-Dextran conjugated Endorem NPs increased the intracellular concentration of the drug compared with the free drug. Endorem is an FDA approved dextran coated iron oxide NP. The Catechin-Dextran-Endorem formulation induced apoptosis in 98% of human pancreatic cancer cell line (MIA PaCa-2) placed under a magnetic field. The findings suggest that conjugation of catechin–dextran with Endorem enhances the anticancer activity of this drug and provides a novel means for targeted drug delivery to tumor cells driven by magnetic fields. The authors concluded that the ability to spatially control the delivery of the catechin–dextran by a magnetic field makes it a promising agent for cancer therapy.108
Often the surface of iron oxide NPs for chemotherapy will also contain a targeting agent which can identify the receptors over-expressed on the external surface of cancer cells.110 Recently Lee et al.111 designed urokinase plasminogen activator receptor (uPAR)-targeted magnetic iron oxide nanoparticles (IONPs) carrying gemcitabine as a chemotherapy drug for targeted delivery into uPAR-expressing tumor and stromal cells. The novel formulation was prepared by conjugating IONPs with the amino-terminal fragment peptide of the receptor-binding domain of uPA. uPA is a naturally occurring ligand of uPAR, and gemcitabine via a lysosomally cleavable tetrapeptide linker. These theranostic nanoparticles were designed to enable intracellular release of gemcitabine following receptor-mediated endocytosis into tumor cells whilst providing contrast enhancement in magnetic resonance imaging (MRI) of tumors the results demonstrated the pH- and lysosomal enzyme-dependent release of gemcitabine, preventing the drug from enzymatic degradation whilst imaging inside the tumor was possible.
Gold coated iron oxide nanoparticles
In order for these MNP’s to be safe they are coated with materials such as silica, polymers or gold. Iron oxide NPs coated with gold are referred to as hybrid nanoparticle HNPs, (Figure 7). Gold surfaces bind strongly with thiol (-SH) containing molecules via dative covalent linkages. This allows for ease of surface modification. Some studies have exploited this for the treatment of pancreatic cancer. Recently, Barnett and colleagues published a study on the attachment of 6-thioguanine onto HNPs.112 6-TG is an antitumor agent used clinically for the treatment of leukaemia.113 6-TG belongs to the thiopurine series of compounds which are renowned for their immunosuppressant properties and are commonly used in organ transplants to reduce rejection of new tissue and also in the treatment of Crohn’s disease.114 In this study, 6-TG was successfully conjugated onto the HNP surface with a ratio of 3:1:10 Fe: Au: 6-TG (wt: wt: wt). After incubation with BxPC-3 cells, enhanced cellular uptake was reported with the 6-TG-conjugated HNPs compared with free drug. A 10-fold decrease in IC50 was also observed highlighting the exciting potential of HNPs for use as drug carriers. Physical conjugation of other anticancer agents such as cisplatin onto HNP surfaces has also been reported.115
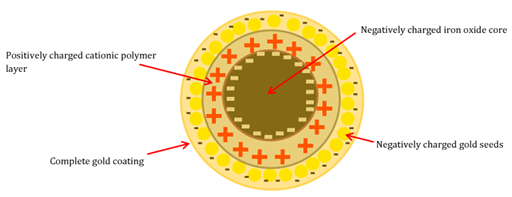
Figure 7 Schematic diagram of gold coated iron oxide nanoparticle showing electrostatic charges within each layer.
HNPs possess dual functionality. As well as retaining the inherent magnetic properties of the iron oxide core they also gain optical properties arising from the gold shell. Colloidal gold possesses unique surface plasmon resonance (SPR) properties. When nanoparticles are irradiated with light of appropriate wavelength, particles undergo absorption and scattering of the photons. This absorption of gold nanoparticles is followed by rapid conversion of light into thermal energy.116 This unique property can be exploited for further applications such as photo thermal ablation and thermo-responsive drug delivery.108 Clinically, the optimal wavelengths for laser irradiation of gold nanoparticles are within the ‘biological near infrared region (NIR)’.117-119 Laser beams inside the NIR window are capable of deep tissue penetration due to the high transmisivity of water and haemoglobin within these wavelengths.120
Recently, Guo and colleagues have reported the photo thermal ablation of human pancreatic cell line (PANC-1). Here they showed that GoldMag® (50 µgmL-1) nanoparticles were capable of cellular internalisation and temperature elevation after near infrared irradiation (7.9 Wcm-1, 5 min). The temperature elevation achieved was as high as 79.51˚C which resulted in only 2.3% cellular proliferation after 24 h compared with 47.0% in the non-irradiated control.121 Use of HNPs for thermo-responsive drug delivery is now a major focus. This technology benefits from magnetic properties reduced toxicities and the ability to target the drug release using focussed laser irradiation. Due to the relative age of this technology to the best of our knowledge no work has yet been published for pancreatic cancer therapy.
Iron oxide incorporated into supramolecular systems
As scientific knowledge increases more sophisticated platforms are emerging. Iron oxide NPs and HNPs have been incorporated into large macromolecular structures to exploit their magnetic potential whilst maintaining the properties of the larger system. One example of this is the fabrication of magneto-liposomes. Here iron oxide is incorporated into the liposome structure (Figure 8). Liposomes are spherical structures composed of a phospholipid bilayer surrounding aqueous reservoir.121,122 Liposome vesicles are composed of unilamellar or multilamellar lipid bilayer which have alternative aqueous layers sandwiched between the bilayer.123 Frascione and colleagues demonstrated the successful incorporation of super paramagnetic iron oxide NPs into a PEG coated liposome. The particle diameter of magnetic liposomes ranged between 100-200 nm. An effective iron oxide loading was achieved, with encapsulation efficiency between 74% and 92%. Their study showed that even after incorporation into larger macromolecular systems the intrinsic magnetic nature of the SPIONs was still capable of giving good contrast-ability on the MRI.
Hydrophilic or hydrophobic drugs can become encapsulated inside the aqueous or lipid phase of the liposomes, respectively.122,124 Liposomes have been shown to improve the therapeutic efficacy of pharmaceutical drugs including ibuprofen, amphotericin B and doxorubicin.122,125-129 Incorporation of iron oxide nanoparticles into the intrinsic liposome structure results in a delivery system capable of real time tracking in vivo. Deng and colleagues fabricated a magnetic liposome structure for pancreatic cancer therapy.130 Here, ultra-small SPIONs were incorporated into a liposome containing doxorubicin forming a theranostic agent. The surface of the liposome was coated with anti-mesothelin antibodies which are over expressed in pancreatic cancer cells and subsequently poly(ethylene glycol) to give stealth properties. The novel formulation was tested on PANC-1 cells. The in vitro studies showed the magnetic liposome’s possessed good relaxivity on the MRI leading to high contrast-ability. The in vivo antitumor study demonstrated that compared with the free drug and the liposomal formulations both with and without SPION incorporation possessed a tumor retardation effect. A tissue distribution assay further proved that magnetic liposomes were capable of selectively accumulating inside the tumor xenograft. These results indicated that the magnetic liposomes not only retained the inherent MRI capability of SPIONs well, but also they significantly improved the distribution of the SPIONs and therapeutic agents in pancreatic tumor tissues.130
A recent study showed successful incorporation of HNPs into the intrinsic structure of a graft amphiphilic polymer derivative of poly(allylamine) (PAA).131 Amphiphilic polymers are comprised of hydrophilic and hydrophobic domains which spontaneously aggregate into core-shell nano-aggregates in aqueous environments. Poly(allylamine) grafted amphiphiles have been reported in the solubilisation of lipophilic drug molecules including propofol, prednisolone, griseofulvin (132) and the novel anticancer therapeutic BNIPDaoct (previously discussed).47 The formation of a polymer-HNP conjugate resulted in a bifunctional platform that can be used for image guided drug delivery via exploitation of the inherent magnetism possessed by the iron oxide core. Here the gold coating of the HNP formed strong dative covalent bonds with the hydrophobic oxadiazole (Ox) pendant group via its thiol (-SH) functionality. The PAA-Ox5 aggregates exploited as drug carriers using both direct conjugation of drug molecules (6-Thioguanine, 6-TG) and encapsulation of hydrophobic drug molecules (novel anticancer agent bisnapthalamidopropyldiaminooctane, BNIPDaoct). In vitro assays carried out using human pancreatic adenocarcinoma (BxPC-3) cells showed that treatment with the polymer-HNP formulation (with both directly conjugated and encapsulated drugs) resulted in an increased drug uptake compared with the free drug. A significant decrease in the IC50 value compared with the free drug was also observed. These observations show that not only is the incorporation of HNPs feasible into amphiphilic polymers but also it can increase their drug solubilisation efficiency whilst adding imaging capability.131
The studies highlighted show the great potential of iron oxide to be utilised in this field. More work needs to be carried out in order to determine to optimise parameters and understanding in areas such as penetration depth, drug efficacy and clearance mechanism of the nanoparticle systems. Long term stability needs to be studied in depth in order to avoid the degradation of iron oxide into free radicals and magnetic clustering causing formulation challenges. The wealth of expertise in this area is likely to overcome these issues resulting in treatment progressing through trials and into the clinic. It is imperative that more efforts focus on pancreatic cancer applications and the advantages gained over using the ranostics in cancer. In pancreatic cancer the time between diagnosis and treatment often is of detriment to the patient. Rapid diagnosis and treatment using iron oxide the ranostics may hold the key to improved clinical outcomes and patient quality of life.
Iron oxide has shown to hold potential as an imaging and chemotherapy for pancreatic cancer. As scientific focus increases into pancreatic cancer, the development of theranostic agents may hold the key to improved treatment of this fatal disease.
None.
None.