eISSN: 2379-6367


Research Article Volume 2 Issue 6
1Department of Tropical Medicine, Medical Microbiology and Pharmacology, University of Hawaii, USA
2Department of Obstetrics, Gynecology and Women's Health, University of Hawaii, USA
Correspondence: Ron Arleigh C. Tamayo, Creighton University, 2500 California Plaza Omaha, NE 68102 USA, Tel 8082589301
Received: January 05, 2015 | Published: November 25, 2015
Citation: Tamayo RAC, Wright TE, Collier AC. Does methamphetamine abuse affect serotonin signaling in the maternal-placental-fetal system? Pharm Pharmacol Int J. 2015;2(6):208-212. DOI: 10.15406/ppij.2015.02.00042
Although methamphetamine (METH) is the most commonly abused drug during pregnancy, there is surprisingly little data on METH effects on gestational tissues. This study, building on our recent research describing the effects of METH on antioxidant status and catecholamine signaling in gestational tissues, assesses serotonin levels in the umbilical cord during pregnancy and effects of demographics and neonatal variables. We hypothesized that METH ingestion and/or smoking may alter serotonin signaling in the umbilical cord. Umbilical cords (n=85) were collected, processed to total lysates, and standardized to 2 mg/mL protein. Serotonin levels were determined by ELISA then correlated to different demographic and pregnancy variables, antioxidant and catecholamine levels then the effects of METH and/or smoking determined. Levels of serotonin in umbilical cords were not significantly different between METH users nor smokers and control cords. Additionally, there were no significant correlations with delivery type, parity, gravidity neonatal baby weight, or maternal age. Serotonin levels were positively correlated with maternal diabetes (P=0.04) and negatively correlated with Gestational age (P=0.05, r=0.2310) and placental weight (P=0.04, r=-0.3527). Serotonin levels may also be affected by maternal hypertension (P=0.06) as well as preeclampsia (P=0.08). While umbilical serotonin was not related to levels of noradrenaline and dopamine nor to the antioxidant enzymes glutathione-S-transferase, catalase, super-oxide dismuatse or xanthine oxidase levels; serotonin increased as thioredoxin reductase (TRX, P=0.0003, r=0.40) and glutathione peroxidase (GPx, P=0. 01, r=0.28) activities increased. A potential association between serotonin and with Vitamin C levels was also noted (P=0.07, r=0.2942). When stratified for METH and smoking, patients with diabetes showed that umbilical serotonin may be further lowered by smoking but not METH (P=0.06 non smokers vs. smokers, P=0.08 smokers vs. Meth+smokers). Furthermore, THC caused a significantly higher gravida (P=0.01) than non-smokers. When stratified, smokers showed a significantly higher chance in getting pregnant than those that do not smoke (P=0.0002). Those that smoke in addition to THC, however, have a significantly lower chance of getting pregnant than those that only smoke (P=0.0173). THC also suggestively causes a lower neonatal baby weight (P=0.02). Whilst THC did not show any significant differences in CAT levels with respect to different types of smokers, those who smoke THC have significantly higher levels of Vitamin C (P=0.0383) ,a significantly lower level of GPx activity than those who do not smoke (P =0.05), and a significantly higher level fof noradrenaline (P=0.02). Serotonin signaling in umbilical cords is affected by hypertension and diabetes as well as oxidative stress, which may itself be caused by the two clinical syndrome.
Keywords: serotonin, fetal, physical abnormalities, phenylephrine
METH, methamphetamine; THC, tetra hydro cannabinol
Any compound ingested during pregnancy can, depending on its physico-chemical characteristics, pass into the fetal circulation. After transiting the placenta in blood, chemicals emerging into the fetal circulation are delivered directly to the fetal heart by the umbilical artery. The fetal heart bypasses the pulmonary circuit and pumps blood around the fetal circulation then returns de-oxygenated blood carrying metabolic wastes to the placenta through the umbilical vein.1
Hence, the umbilical cord plays a vital role in both delivering and removing oxygen, essential nutrients and other chemicals to and from the fetus. Therefore, the placental and umbilical vessels are, also the major route for harmful drugs and chemicals to reach the fetus. These harmful chemicals may cause direct deleterious fetal effects and may also interfere with placental or umbilical function thereby disrupting with the delivery and removal of blood and other essential substances.1
Most physical birth defects occur during the embryonic period (up to 8weeks from conception)2 when the embryo is differentiating and organ structure developing.3 Also during the embryonic period, the umbilical cord and placenta are not fully functional and cannot detoxify substances transiting from the maternal circulation.3 After the embryonic period, physical abnormalities of teeth, palate and ears can occur up to 16weeks.2 Furthermore, functional and physical abnormalities of the eyes, central nervous system and genitalia can be caused at any time from conception to term.2
Most women do not know that they are pregnant until the second missed menstrual cycle, hence; often the embryonic period has passed without any conscious knowledge or change in behavior in the mother.4 Consequently many women accidentally continue recreational or medical drug use and keep ingesting foods and other substances that they would otherwise to avoid. In other cases, some mothers either do not (by choice) or cannot (through addiction or medical need) stop their ingestion of potentially harmful substances.4 Methamphetamine (METH), and to a lesser extent Marijuana addictions belong to the latter case where, more often than not, the mother is physically and/or psychologically unable to stop ingesting these drugs. METH use in pregnancy is associated with a number of substandard reproductive and pregnancy outcomes including pre-eclampsia, pre-term birth, intra-uterine growth restriction, small-for-gestational-age babies and neonatal addicts. Other studies have reported that marijuana use in pregnancy is also associated with low birth weight and small for gestational age infant, as well as preterm delivery; other relationships involve affecting the neural and morphological development of the fetus.5
Both METH and the active ingredient in marijuana (D9-tetrahydrocannabinol, THC) have interactions with serotonin receptors and serotoneergic signaling. As early as 1974 interactions between amphetamine effects and serotonergic signaling were being reported.5 Amphetamines are potent inhibitors of 5HT binding in the brain6 but little is known about METH and 5HT interactions in the maternal-placental-fetal unit. The one study in this area demonstrated that amphetamines can inhibit placental monoamine transporters7 but there is a dearth of knowledge regarding placental, umbilical or fetal interactions between METH and 5HT. Similarly, it has been shown that cannabinoids can directly inhibit the 5HT3 and 5HT1a receptors8,9 and that patients with a clinical deficiency in endocannabinoids and, to a lesser extent cross talk between THC and 5HT signaling, may explain therapeutic benefits of marijuana use in some syndromes.10 However, investigations of the effects of THC on umbilical cord serotonin levels and pregnancy outcomes are likewise, absent from the literature.
It has long been known that serotonin and other compounds that bind serotonin receptors (such as histamine) an alter tone in the umbilical artery and vein.11 The human umbilical artery and vein have both have been reported to express 5HT1D beta, 5-HT2A, 5-HT2B, 5-HT4, and 5-HT7.12,13 It has subsequently been demonstrated that only 5HT1B/D and 5HT2A cause functional contraction of the human umbilical artery and vein.14–16 Serotonin is of interest for altering umbilical contractility since studies have shown that noradrenaline, the predominant sympathetic neuro transmitter, as well as phenylephrine and clonidine can only cause contraction in the umbilical vein of 9%, 14% and 11% respectively of the maximum caused by 5-HT.16 In the umbilical artery, the maximalcontractility caused by noradrenaline and phenylephrine was higher, but still only 18% and 29% of that for 5-HT, and clonidine had no effect.16 Additionally, histamine and 5HT produced strong, dose-dependent vasoconstrictions but epinephrine and norepinephrine cause only slight vasoconstriction.17 Meanwhile early studies also demonstrated that the human fetal heart can respond to serotonin18 Subsequently, 5HT2B and 5HT4 receptors have been identified in fetal heart structures.19,20 Evidence suggests that the responses of the umbilical endothelia to 5HT are oxygen-dependent and may only occur under conditions of low oxygen tension.21 This raises interesting questions for pregnancy conditions where oxidative stress may be present such as during pre-eclampsia and/or when medial or illicit drugs with highly oxidative properties (such as COX-2 inhibitors or METH) are ingested during pregnancy.
In addition to providing a conduit for METH to directly reach the fetus, changes in vascular tone in the umbilicus may adversely affect fetal development by restricting the delivery of essential compounds and/or removal of wastes. Secondly, since the umbilical artery leads directly to the heart, if METH causes high levels of catecholamines to be produced in the umbilical artery, may adversely affect the fetal cardiovascular system. Whilst the net effect of this is unlikely to alter fetal circulatory dynamics, since changes in heart muscle dynamics tend to be balanced by changes in vascular tone.22 The implications of higher levels of catecholamines produced in the umbilical artery and delivered directly to the fetal heart include increased work by the heart and the potential for dysrhythmias, which may cause fetal distress and/or death. Finally, since catecholamines affect gland cells and change neuro-endocrine signaling22 METH may affect normal endocrine signaling in the developing fetus, either through direct delivery of METH to fetal endocrine tissues or through increased catecholamine release in the umbilical cord which are then delivered to fetal tissues. In this paper we have assessed the effects of METH ingestion, Marijuana and tobacco smoking, independently and together on serotonin in umbilical endothelia. We hypothesized that ingestion of METH, THC and/or tobacco smoke may cause increased production of serotonin or alter serotonergic signaling in the umbilical vessels.
Tissue collection and processing
One hundred umbilical cord pieces (10cm) were collected with informed consent from women undergoing Caesarian section at term. 85 were available for this study. Each piece of cord was coded with a study number, then stored at -80ºC until use. This study was approved by the University of Hawaii Institutional Review Board for Human Subjects and the Kapiolani Hospital Ethical Review Board.
For processing to lysates, cord pieces were thawed, wet weight recorded then cords homogenized mechanically with an IKA Labortechnik Laboratory homogenizer (XXX, Germany) at 6,500 RPM for 30 s each in a 1:4 (w:v) Tris-HCl buffer containing 5mM MgCl2 and 2mM PMSF (pH 7.4). Total lysates were normalized to 2mg/mL protein using the Bicinchoninic acid method, then aliquoted and frozen at -80°C until use.23
ELISA for catecholamine neurotransmitters and serotonin. Levels of serotonin were determined with a commercial Serotonin ELISA from Rocky Mountain Diagnostics (Colorado Springs, CO) as per the manufacturer’s instructions.
Data analysis and statistics
Quantification of serotonin was performed blinded using only the study number for identification of samples. After ELISAs were completed and concentrations assigned, the study key was accessed and results were sorted into four groups: cords from non-smoking, non-METH patients (N), cords from METH using patients (M), cords from tobacco smoking patients (S), cords from THC positive patients (T), and cords from patients who both smoked tobacco and used METH (T/M), cords from those who both smoked tobacco and were THC positive (T/S) and cords from mothers who used all three (M/T/S). Data on METH and tobacco status were based on self reported METH or smoking status and were also confirmed with ELISA detection of METH parent drug and metabolites and/or cotinine levels in umbilical cord lysates.24 Statistical analyses were performed using Prism 5.0 with significance set at α=0.05. (GraphPad Prism, San DiegoCA). Since these data are discrete, two-tailed student t-tests were used to assess differences between groups. Stratification and comparisons between groups were analyzed using multi-variate analyses.25
When levels of serotonin in umbilical cords were compared with demographic variables, there were no significant differences between METH users nor smokers and control cords (figure not shown). Additionally, there were no significant correlations with delivery type, parity, gravidity, neonatal baby weight, or maternal age (figure not shown). Serotonin levels were, however, positively correlated with maternal diabetes (P=0.04) and negatively correlated with Gestational age (P=0.05, r=0.2310; Figure 1A, Figure 2A, respectively). Moreover, Serotonin levels were negatively correlated to placental weight (P=0.04, r=0.3527; Figure 2B). Serotonin levels may also be affected by maternal hypertension (P=0.06) as well as Preeclampsia (P=0.08; Figure 1B, Figure 1C, respectively). Serotonin levels are suggestively not related to levels of noradrenaline and dopamine nor to antioxidant enzymes glutathione-S-transferase, catalase, super-oxide dismutase, or xanthine oxidase levels (figures not shown); serotonin increased as thioredoxin reductase (TRX, P=0.0003, r=0.40) and glutathione peroxidase (GPx, P=0.01, r=0.28) activities increased (Figure 2D, Figure 2E, respectively). There is also a potential association between serotonin and with Vitamin C levels and serotonin levels (P=0.07, r=0.2942; Figure 2C). When stratified for METH and smoking, patients with diabetes showed that umbilical serotonin may be further lowered by smoking but not METH (P=0.06 non smokers vs. smokers, P=0.08 smokers vs. Meth+smokers; figures not shown). Furthermore, THC caused a significantly higher gravida (P =0.01) than non-smokers (Figure 3). When stratified, smokers showed a significantly higher chance in getting pregnant than those who do not smoke (P=0.0002; Figure 4). Those that smoke in additiont o THC, however, have a significantly lower chance of getting pregnant than those that only smoke (P=0.0173, Figure 4). THC also suggestively causes a lower neonatal baby weight (P=0.02, Figure 5). Whilst THC did not show any significant differences in CAT levels with respect to different types of smokers, those who smoke THC have significantly higher levels of Vitamin C (P=0.0383, Figure 6), a significantly lower level of GPx activity than those who do not smoke (P=0.05, Figure 6), and a significantly higher level of noradrenaline (P=0.02, Figure 7). Serotonin signaling in umbilical cords is affected by hypertension and diabetes as well as oxidative stress, which may itself be caused by the two clinical syndromes.
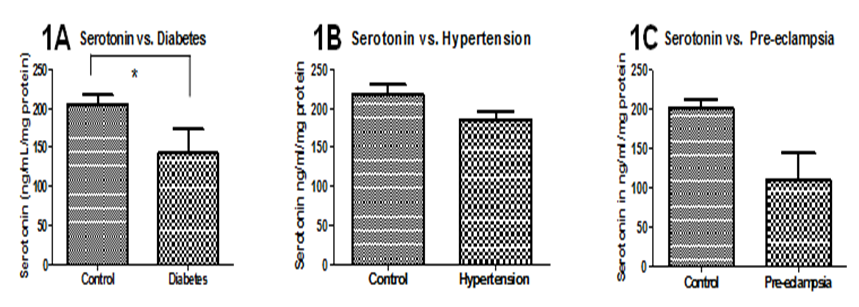
Figure 1 Associations between serotonin levels and demographic, clinical variables. 1A: 1A: Serotonin levels are significantly lower in those that have diabetes (P=0.0472). 1B, 1C: Serotonin levels may be lower in those that have hypertension (P=0.0604) and pre-eclampsia (P=0.0844). There are no significant differences when samples are stratified into different types of smokers (not shown). Significance was assigned using T-tests (α=0.05). *=P<0.05, **=P<0.01, *** P<0.001. Bars are means ± SEM.
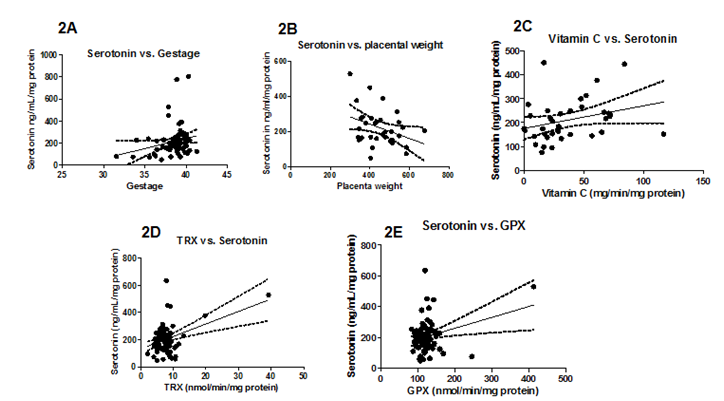
Figure 2 Serotonin levels in the Antioxidant Defense Network and Pregnancy variables. 2A, 2D, 2E: Gestation (P =0.05), TRx (P=0.0003), and GPx (P=0.0132) levels increase with serotonin levels. 2B: Serotonin levels significantly decrease as placental weight increases (P=0.0441). 2C: Vitamin C levels may increase with serotonin levels (P=0.0772). Significance was assigned using correlation, linear regression P values, and 95% Confidence lines.
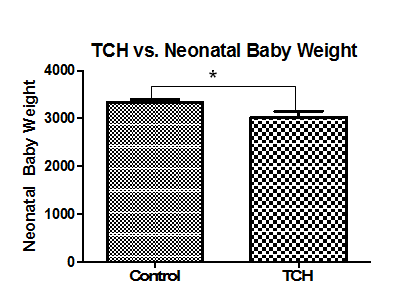
Figure 3 Gravida levels with respect to TCH. Those who smoke THC have a significantly higher chance of getting pregnant than those who do not smoke THC (P=0.0149).Significance was assigned using T-tests (α=0.05). *=P<0.05, **=P<0.01, *** P<0.001. Bars are means ± SEM.

Figure 4 Gravida and different types of smokers. Those that smoke a significantly higher chance in getting pregnant than those that do not smoke (P=0.0002). Those that smoke in addition to THC have a significantly lower chance of getting pregnant than those that only smoke (P=0.0173). Significance was assigned using T-tests (α=0.05). *=P<0.05, **=P<0.01, *** P<0.001. Bars are means ± SEM.
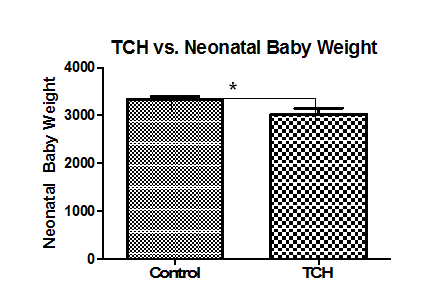
Figure 5 THC and neonatal baby weight. Neonatal baby weights are significantly lower in those that smoke THC (P=0.0246) than those that do not smoke at all. Significance was assigned using T-tests (α=0.05). *=P<0.05, **=P<0.01, *** P<0.001. Bars are means ± SEM.
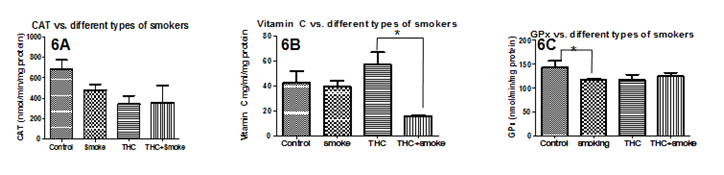
Figure 6 Antioxidant Defense Network enzymes versus different types of smokers. 3A: There are no significant differences in CAT levels with respect to different types of smokers. 3B:Those who smoke THC have significantly higher levels of Vitamin C (P=0.0383). 3C: Those who smoke have a significantly lower level of GPx activity than those who do not smoke (P =0.05). Significance was assigned using multiple t-tests (α=0.05). *=P<0.05, **=P<0.01, *** P<0.001. Bars are means ± SEM.

Figure 7 GST, SOD, and NA levels versus different types of smokers. 4A: There are no significant differences in GST levels with respect to different types of smokers. 4B:Those who smoke have significantly higher levels of SOD (P=0.0023). 4C: Those who smoke THC and other illicit drugs have significantly higher levels of noradrenaline (P=0.0217). Significance was assigned using T-tests (α=0.05). *=P<0.05, **=P<0.01, *** P<0.001. Bars are means ± SEM.
None.
The author declares no conflict of interest.

©2015 Tamayo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.