eISSN: 2377-4304


Case Report Volume 14 Issue 2
1Sidi Mohamed Ben Abdellah University, CHU Hassan II Fes, Doctor of Medicine, Served in Gynecology and Obstetrics, Morocco
2Professor of Graduate Studies; Gynecology and Obstetrics department 2 CHU Hassan II Fes, Sidi Mohamed Ben Abdellah University of Fes, Professor of Higher Education and Head of the Gynecology and Obstetrics Department 2 CHU Hassan II Fes, Morocco
Correspondence: Belhaj yassine, Resident of Gynecology and Obstetrics, Sidi Mohamed Ben Abdellah University, Department of Gynecology – Obstetrics II, HASSAN II University Hospital of Fez, Morocco, Tel +212677629354
Received: March 01, 2023 | Published: March 31, 2023
Citation: Belhaj Y, Kenza B, Alaoui FZF, et al. Boring form of an invasive mole: case report and review of the literature. Obstet Gynecol Int J. 2023;14(2):54-57. DOI: 10.15406/ogij.2023.14.00693
Gestational trophoblastic diseases include a heterogeneous group of pathologies due to abnormal trophoblast proliferation. These diseases are rare and most often have a good prognosis. Management recommendations are based mainly on expert opinions, such as those published in 2020 by the European Organisation for the Treatment of Trophoblastic Disease (EOTTD).
They have a common name, but their origins, clinical characteristics and treatment differ.
Hydatidiform moles correspond to villi with an excess of paternal genetic material and having a malignant potential, higher for complete Hydatidiform moles than for partial Hydatidiform moles
Invasive mole is responsible for most cases of localized gestational trophoblastic neoplasia, occurring during pregnancy, within a variable period, but in most cases it is diagnosed after a molar pregnancy.
Histological proof is sometimes difficult on curettage product, and must then take into account imaging and serum hCG levels.
The overall cure rate is nearly 100% in low-risk patients and 90% in high-risk patients, since the advent of chemotherapy.
In rare cases, the molar tissue crosses the thickness of the myometrium, the serosa and leads to hemoperitoneum associated with an acute abdomen as well as metastases, which is infrequently encountered in the invasive mole.
The best course of treatment is chemotherapy (depending on the stage and the score with mono or poly chemotherapy) and in patients whose fertility is not in question, a hysterectomy can be performed.
On the occasion of a clinical case, we will expose a dissecting form with an ovarian metastasis of an invasive mole. The treatment was successful. During the follow-up, she remained free of the disease without any sequelae.
Keywords: gestational trophoblastic neoplasia, hemoperitoneum, invasive mole, trophoblast, dissecting form
The invasive mole arises from myometrial invasion of the Hydatidiform mole through direct extension through the tissue or venous system. It is characterized by edematous chorionic villi with trophoblastic proliferation that invades directly into the myometrium.1–3 Locally invasive gestational trophoblastic neoplasia develops in 15% of patients and a metastatic form in 4% of patients after evacuation of a complete mole and more rarely after a partial mole.4
HCG level (>100,000mIU/ml), globose uterus, luteal cyst size ≥6cm are considered high risks for developing post-molar tumors (high-risk form).5 The most usual symptomatology of the invasive mole is the metrorrhagia of the 1st trimester is persistent after the evacuation of the molar pregnancy associated with the non-involution of the uterus and persistence of the cyst of the body of the theca lutein. The increase in the titer of βHCG is of interest for the diagnosis of invasive mole in the follow-up of molar pregnancy. Definitive diagnosis of the invasive form is obviously based on pathology,6 however persistent trophoblastic disease (PTD) is a clinical and non-histopathological diagnosis and the role of
In rare cases, the invasive mole can be metastatic and the most frequent sites encountered are: the lungs (80%),2 the vagina (30%), the pelvis (20%), the liver (10%), brain (10%), intestine and kidney and spleen (<5%) were the other metastatic sites.
Ovarian metastases from an invasive mole are, however, rare, indeed about 5 to 6% of ovarian tumors come from metastases from other organs.
The possibility of metastasis to the ovary is extremely rare, which is even much lower than that of non-gestational primary ovarian choriocarcinoma whose incidence is 1 in 3.7×108.7 Non-gestational ovarian choriocarcinoma is a major differential diagnosis. Metastases to the omentum are very rare in cases of invasive mole. The invasive mole is curable with chemotherapy but the surgical treatment (hysterectomy) limits the use of several cures. Additionally, in patients with heavy bleeding or sepsis, chemotherapy is necessary to stabilize the patient.8
We report the case of a borer form of invasive mole having overflowed the serosa with invasion of the ovary. Multidisciplinary oncologist and gynecologist management including hysterectomy and mono-chemotherapy was essential.
Our patient is 47 years old, she is a housewife, with a living child gives birth by high way, currently 4 years ago, admitted for low to medium metrorrhagia, associated with pelvic pain on an amenorrhea of 16 weeks. In whom the general clinical examination finds a conscious patient, stable on the HD and respiratory levels, normotense, with a heart rate of 100 beats/min, slightly discolored conjunctiva, apyretic eupnea. With the abdominopelvic examination, a soft but sensitive abdomen a normal-looking vulva, and speculum: a gravid purplish cervix, low-abundance bleeding from the endocervix, on vaginal examination couple on abdominal palpation: an enlarged uterus, sensitive to mobilization.
On a biological level, an HCG level of 124,856, then, the pelvic ultrasound showed an enlarged uterus with a hyper echogenic heterogeneous honeycomb image of 43x31mm, non-vascularized on Doppler, in favor of a Hydatidiform mole. With the presence at the fundal level of 2 heterogeneous images measuring 55x32mm and 20x16mm at the peripheral level, raising doubts about myometrial invasion, hence the realization of cross-sectional imaging to assess loco-regional extension (Figures 1–4).
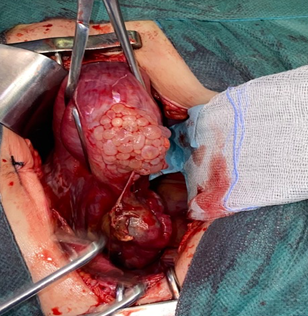
Figure 1 Intraoperative image showing the presence of molar vesicles exceeding the serosa of the globular uterus, with a heterogeneous-looking ovary.

Figure 2 According to the FIGO Score: age >40 years: 1, atcd of pregnancy: mole: 0, interval between pregnancy and start of treatment: < 4 months: 0, beta HCG: 12,000 i.e. score 2, large tumor size: >5 or 2, site of metastasis: lung therefore: 0, 1 lesions on the metastasis between 1-4:1.
Score thus: 6. Low risk➮mono chemotherapy
A score or equal to 6: low risk A score > or equal to 7: high risk Tumors at the implantation site are excluded from this score.
The total score is obtained by adding the individual scores for each prognostic variable. Count all metastases, not sites.
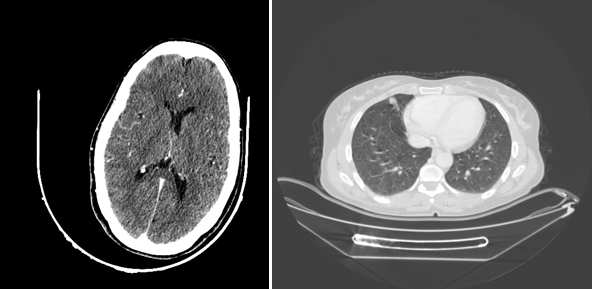
Figure 3 Scanographic image: on the left at the cerebral level: a cross section of the C+ skull not showing an image related to a secondary location, unlike the thoracic level: showing a right lower lobe nodule.
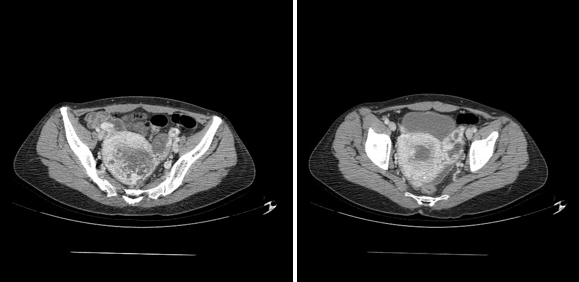
Figure 4 MRI image showing on the right a uterus increasing in size site of a heterogeneous intracavitary image extended to the left ovary.
And so, the pelvic MRI was in favor of a gestational trophoblastic tumor invading the serosa coming into contact with the ovary and the rectum posteriorly measuring 65*45mm associated with thrombosis of the left internal iliac vein and the homolate ovarian vein, just as a Hemoperitoneum of low abundance (Figure 4).
Then, a TAP CT scan looking for distant metastasis demonstrated the presence of pulmonary nodules in the upper and middle right lobes related to secondary locations (Figure 3).
Patient benefited from a total hysterectomy with bilateral adnexectomy given the significant risk of uterine rupture if chemotherapy first.
In the exploration illustrated in Figure 1; it is a uterus increasing in size making 12 weeks of amenorrhea, with the presence of vesicles at the level of the serous reminiscent of the molar vesicles and a local extension towards the ovary of macroscopically heterogeneous aspect, in the company of a low abundance hemoperitoneum aspirates estimates at 200c.
Our patient was staffed in a multidisciplinary meeting, classed according to the FIGO score with a decision to supplement with mono chemotherapy.
Gestational trophoblastic diseases (GTD) represent a class of rare conditions that are characterized by abnormal trophoblastic proliferation. The most common GTD is hydatidiform mole (HM), a conceptus with a frequency of 1–2/1000 pregnancies.9
In fact, the incidence of GTT is higher in patients aged over 40, as was illustrated10 from an analysis of 18 international studies incriminating advanced maternal age as a major risk factor for GTT. The relative risk multiplies by 7.8 after 40 years, this can be explained by genetic factors, associated with the aging of the oocyte, and also by a deficiency in carotene and vitamin A, which favor fertilization anomalies and by a lower maternal immunological reaction.10 However, some authors do not consider blood group A as a risk factor; it is rather ABO blood incompatibility between the mother and the father which is incriminated, through immunological factors.9 Our patient was 47 years old with blood type A.
In addition, GTTs can follow any state of pregnancy in 50% after molar pregnancy, 25% after abortion and 25% after pregnancy carried to term.9 In our case, it is a complete softness which is complicated in TTG.
The most frequent diagnostic circumstances of GTT were represented by the appearance of metrorrhagia in half of the cases, and/or disturbance of the serum BHCG level during post molar follow-up.
The delay between causal pregnancy and diagnosis of GTT, six months found in the literature.11,12 This justifies the interest of close monitoring of patients during the 12 months following the molar abortion, the risk being much lower beyond 12 months.12,13
FIGO published an update published in 2007 on compliance with diagnostic criteria.
Sonographic imaging in GTN can present with a variety of different features so there is no typical sonographic and Doppler characteristics of GTN; therefore, correlation with hCG levels is vital to establish a differential diagnosis with other conditions that can mimic this diseases, for instance such pelvic inflammatory disease, arteriovenous malformations, or other uterine malignancies.14
Although, the classical ultrasound finding of gestational trophoblastic disease presents of “snowstorm” or “honeycomb” appearance where the presence of the multicystic lesion in the uterine cavity.15
Metastatic disease workup can involve various modalities, including ultrasound, X-ray, TDM, IRM and the pet scanner.14 In our patient, a pulmonary nodule of metastatic origin was objectified on chest CT and chest X-ray.
On the therapeutic side, thanks to the development of chemotherapy in the management of TTG, the cure rate has continued to increase.
Methotrexate as mono-chemotherapy is the first-line treatment for low-risk forms (FIGO score <6).11,12
The administration of methotrexate is done in our department at the rate of 1mg/kg on D1, D3, D5, D7 repeated every 14 days, until normalization of HCG by adding 3 cures after negativation. In combination with folic acid to reduce the necessary number of cures.10
Currently, the excellent chemo-sensitivity of these tumors has reduced the place of surgery. However, some surgical indications persist.16 like the case of our patient whose file was staffed in CPR with a decision to perform a hysterectomy given the high risk of rupture in the terebrant form with methotrexate.
In fact, Hysterectomy may be required in invasive mole in order to control vaginal bleeding and in unstable patient or in sepsisis, also for patients who do not wish to preserve their fertility. Furthermore, in patients with extensive uterine tumor, hysterectomy may substantially reduce the trophoblastic tumor burden and thereby limit the need for multiple courses of chemotherapy.2
As GTTs are not hormone-dependent and ovarian metastases are rare, the ovaries can be preserved depending on the patient's age.16 In our patient, an ovarian metastasis by contiguity was objective.
Radiotherapy has a limited role10 in the management of GTT, especially as palliative treatment in the event of cerebral metastases, hepatic metastases to reduce the risk of hemorrhage; and vaginal metastases.
The monitoring protocol adapted by FIGO is based on the clinic, one pelvic ultrasound if uterine tumor and others depending on the presenting sign. And also monitoring is based on repeated dosages of serum beta hCG, according to a particular rhythm; while keeping patients on effective contraception to prevent pregnancy from interfering with the B-hcg assay.10,12
Patients treated for TTG can retain fertility, and pregnancy is allowed one year after the end of treatment, to allow proper monitoring of B-hcg and prevent the teratogenic effect of chemotherapy, the hCG levels should be measured 6 weeks postpartum and the placenta sent for pathology.10,17–19
Hydatidiform moles (MH) are genetically abnormal conceptions characterized by abnormal chorionic villi, trophoblastic hyperplasia, poor fetal development, and increased risk of developing malignant diseases. It requires a PEC in collaboration between gynecologists, pathologists, oncologists as well as an awareness of the personnel, medical and paramedical on the importance of an early diagnosis of this entity in prevention of a diagnosis at the metastatic stage.
In our context, the great difficulty of monitoring always because of the material constraints, and also the absence until now of a codified strategy of prevention. Would it be interesting to create a reference center for trophoblastic diseases in our country?
None.
None.
There is no competing interests between the authors.

©2023 Belhaj, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.