MOJ
eISSN: 2379-6294


Review Article Volume 3 Issue 7
Institute of Zoology of NAS of Azerbaijan, Azerbaijan
Correspondence: Topchiyeva ShA, Institute of Zoology of NAS of Azerbaijan, Baku, AZ1073, Baku, Sabail, Abbasgulu Abbaszadeh, 115, Azerbaijan
Received: December 11, 2017 | Published: December 19, 2017
Citation: Mammadova FZ, Topchiyeva SA. Isolation and identification of biologically active components from the honey bee venom apis mellifera l. caucasica. MOJ Toxicol. 2017;3(7):178–181. DOI: 10.15406/mojt.2017.03.00078
The article presents experimental data on the separation and identification of biologically active components of the venom of the honey bee Apis mellifera L. Caucasica. By gel chromatography on a column with Servacel-52 eluting with a linear gradient of NaCl concentration from 0.01 to 1M, followed by spectrophotometric measurement of the unit of optical density at an absorption wavelength of l=280nm on a Hitachi-557 spectrophotometer from the honey bee venom, biologically active components of zootoxin with molecular weights of 12000, 14600 and 22000D. A polypeptide with Mg~12000D is a toxic component identified as mellitine, a polypeptide with Mg~14600 is an enzyme of phospholipase A2, and a polypeptide with Mg~22000D is a glycoprotein.
Keywords: venom, honey bee, apis mellifera l. caucasica, spectrophotometer, peptides, enzymes
The venom of the European honey bee Apis mellifera is an intricate mixture of chemical compositions, including proteins, peptides, enzymes, and other small molecules. Lately, there has been growing interest in the use of melittin, due to its wide range of biological and potential therapeutic applications. Melittin, which is considered to be an antimicrobial, antitumor, and anti-inflammatory peptide, is the main component (≥50% (w/w)) of honey bee venom and is widely used in oriental medicine1 and studied as an alternative for treating drug-resistant infections.2-4 In parallel to antimicrobial peptides for therapeutic use in humans, melittin can be used to fight economically important plant pathogens that limit crop production globally.5 Phospholipase A2 (PLA2) and hyaluronidase (HYA) are the two major enzymatic proteins present in the bee venom.6,7 Both of these enzymes are classified as major allergens according to the International Union of Immunological Societies, as they are capable of inducing the IgE response in susceptible individuals.6-8
The results of study confirm that a high purity and recovery yield of melittin can be obtained with a one-step purification method with strong cationexchange chromatography resins using sodium phosphate buffer at pH 6. The optimal step-wise elution condition was designed to increase the yield and concentration of the melittin collected. An increased mass of crude bee venom for purification of melittin was also made possible with the optimal condition used in this study. Study has proved to solve the problem whereby melittin can be highly recovered with its allergenic contaminants such as PLA2 and HYA removed in a single-step purification The concern over the use of melittin in honey bee venom due to its adverse reaction caused by allergens such as phospholipase A2 (PLA2) and hyaluronidase (HYA) has been an obstacle towards its usage. Authors developed a novel single-step method for melittin purification and the removal of PLA2 and HYA. This study explores the influence of pH, buffer compositions, salt concentration, and types of cation-exchange chromatography resins on the recovery of melittin and the removal of both HYA and PLA2. Melittin was readily purified with a strong cation-exchange resin at pH 6.0 with sodium phosphate buffer. It resulted in a recovery yield of melittin up to 93% (5.87mg from a total of 6.32mg of initial melittin in crude bee venom), which is higher than any previously reported studies on melittin purification. PLA2 (99%) and HYA (96%) were also successfully removed. Melittin with a high removal rate of PLA2 and HYA, enabling to be fully utilized for its therapeutic purposes.9
Mellitin is the main cytolytic linear peptide with a molecular weight of 2.8 kDa (26 amino acid residues), isolated from the honey bee venom and possessing the properties of a surfactant. Melittin causes hemolysis of erythrocytes, releases histamine from mast cells. The peptide also increases the fluidity of the phospholipid matrix of the membranes, which leads to a change in the activity of many membrane-bound enzymes. The physicochemical properties of melitin cause its pronounced antibacterial activity against many species of microorganisms, including mycoplasma. Phospholipase A2 (FLA2) is a calcium-dependent enzyme. The enzyme has a molecular weight of 14629 Da and consists of 129 amino acid residues, of which 12 are cysteine, which enter the disulfide bridges. It is capable of hydrolyzing phospholipids, resulting in the formation of lysolecithin, which has a cytolytic effect. It has the ability to lyse membranes of many cells (erythrocytes, mast cells), thereby providing a manifestation of pathological effects. In the presence of melitin, the phospholipase becomes more active and toxic. It is suggested that melitin prepares phospholipids for enzymatic activity of phospholipase by its reduced surface tension. Of all the components of bee venom, phospholipase is the strongest antigenic and allergenic protein. Melitin has a hemolytic, antiviral, antifungal and cytolytic effect, causing the death of cells and their organelles. In view of the high cytotoxic and hemolytic activity of melitin, work is underway for its practical use in oncology.10
Like other components of the bee venom: hyaluronidases, apamines, acid phosphatases, etc., melitin also causes degranulation of mast cells and cytotoxicity. Unlike melitin, FLA2 causes only degranulation, cytotoxic effects. Bites of bees can cause life-threatening, and sometimes lethal, antigen-dependent anaphylactic reactions in the human body.11 See venom contains specific protease. It consists about 0.8–15 % of total proteine-peptide fraction. The enzyme was purified with the help of benzamedine-sepharose and reverse-fase chromatography to the activity 110 U/mg. The protein is hydrophobic, its Mr is equal to 20kDa (by gel-filtration), pHopt 4.5. Protease specifically binds to cell membrane in cooperative manner; the maximal binding ratio is 1.2. Enzyme has high substrate specifity for membrane proteins. It is concluded that protease is a specific toxic component of bee venom.12 The molar mass of mellitine is 2840, but the molecular weight determined by analytical ultracentrifugation is 12, 500, which agrees with the tetrameric form in a concentrated aqueous solution of high ionic strength (conditions used for ultracentrifugation). The molar mass of melittin is 2840 but the molecular weight, determined by analytical ultracentrifugation, is 12,500, which is consistent with a tetrameric form in concentrated aqueous solution at high ionic strength, (the conditions used for ultracentrifugation). The peptide acts synergistically with phospholipase A2 on phospholipid structures, whether of natural membranes or liposomes. Although considerably smaller than phospholipase A2 (molar mass 17,500), separation of the peptide (about 40-50%w/w of bee venom) from the enzyme is complicated by the tendency of the peptide to aggregate.13 In connection with the foregoing, there is a need to isolate the biologically active components of the venom of the honey bee, followed by a study of physicochemical properties and biological activity. The aim of the research was the separation, isolation and identification of the main biologically active components of the venom of the honey bee Apis mellifera L. Caucasica.
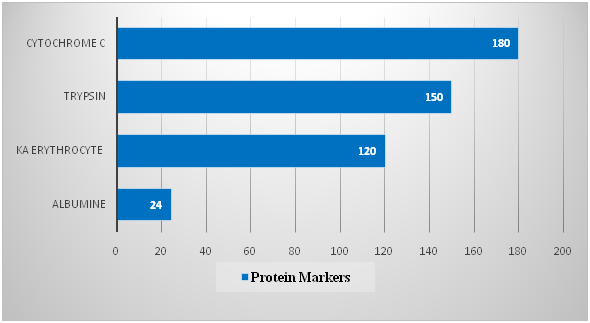
The material of the studies was the whole poison of the honey bee Apis mellifera L. Caucasica. To separate the bee venom components, an ion-exchange chromatography method was used in glass chromatographic columns. Servacel DEAE-52 (Budapest, Hungary) was used as the adsorbent. To construct the calibration curve, the following marker proteins were used: cytochrome C (Mz 12kD), trypsin (20kD), erythrocyte spacecraft (30kD), and albumin lyophilized from human serum (67kD). One division was started by 4 proteins, 2mg each the fractions were collected in 3ml and the optical density was measured on a spectrophotometer. The spectrophotometric determination data are presented in Table 1 and in Figure 1.
No. of Fractions |
Protein Markers |
VR, Ml |
MM, Kda |
1 |
Albumine |
24 |
67 |
2 |
KA Erythrocyte |
120 |
30 |
3 |
Trypsin |
150 |
20.1 |
4 |
Cytochrome C |
180 |
12 |
Table 1 The separation of marker proteins by gel filtration on a column with Servacel 52
In a subsequent stage of the study, the dried crude bee venom (40mg) was dissolved in 2.0 ml of water. Since the bee venom readily dissolves in water and the insoluble foreign matter remains undissolved, for a preliminary purification this poison solution is centrifuged at 6,000rpm for 15min at 4 °C. The supernatant was then separated, followed by filtration, passing the solution through a glass filter with a pore size of 0.20μm and 0.45μm (Millipore, USA). The poison solution was prepared immediately before separation. To separate the components of the poison, the resulting solution in a volume of 1ml was mixed with an equal volume of 1ml of buffer to a total volume of 2ml, and layered on a Servacel-52 column. Samples were chromatographed at room temperature at a flow rate of 3ml/min. Once the sample was loaded onto the column, the column was washed with a buffer solution to remove unbound samples. Elution was carried out using an elution buffer with a linear gradient of NaCl concentration of 0.01 to 1M to ensure effective elution of the components of the honey bee venom. The optical density of the fractions was measured on a Hitachi-557 spectrophotometer at an absorption wavelength at l=280 nm. The fractions were collected in a volume of 3ml (Table 2). The separation of bee venom proteins by the ion-exchange chromatography-graphite method by stepwise elution in a concentration gradient of 0.01-1M sodium chloride solution is shown in Figure 2. The separation of bee venom proteins by gel chromatography on a column with Sephadex G-75 is shown in Table 3 & Figure 3. Comparing the data obtained by us with data from literature sources, it can be stated that we identified and identified mellitine, glycoprotein and phospholipase A2 with molecular weights of 12,000, 14,600 and 22,000D, respectively.15,16
No. of Fractions |
The Unit of Optical Density of Fractions |
No. of Fractions |
The Unit of Optical Density of Fractions |
No. of Fractions |
The Unit of Optical Density of Fractions |
1 |
0.010 |
25 |
0.009 |
49 |
0.152 |
2 |
0.101 |
26 |
0.128 |
50 |
0.350 |
3 |
0.050 |
27 |
0.104 |
51 |
0.012 |
4 |
0.035 |
28 |
0.155 |
52 |
0.006 |
5 |
0.199 |
29 |
0.113 |
53 |
0.011 |
6 |
0.104 |
30 |
0.025 |
54 |
0.004 |
7 |
0.006 |
31 |
0.015 |
55 |
0.009 |
8 |
0.026 |
32 |
0.030 |
56 |
0.250 |
9 |
0.004 |
33 |
0.020 |
57 |
0.011 |
10 |
0.190 |
34 |
0.105 |
58 |
0.002 |
11 |
0.080 |
35 |
0.100 |
59 |
0.001 |
12 |
0.020 |
36 |
0.024 |
60 |
0.460 |
13 |
0.081 |
37 |
0.007 |
61 |
0.011 |
14 |
0.100 |
38 |
0.115 |
62 |
0.002 |
15 |
0.004 |
39 |
0.008 |
63 |
0.001 |
16 |
0.129 |
40 |
0.105 |
- |
- |
17 |
0.003 |
41 |
0.045 |
- |
- |
18 |
0.158 |
42 |
0.095 |
- |
- |
19 |
0.08 |
43 |
0.050 |
- |
- |
20 |
0.007 |
44 |
0.016 |
- |
- |
21 |
0.020 |
45 |
0.010 |
- |
- |
22 |
0.065 |
46 |
0.009 |
- |
- |
23 |
0.100 |
47 |
0.006 |
- |
- |
24 |
0.077 |
48 |
0.004 |
- |
- |
Table 2 Optical density of bee venom fractions, separated by ion exchange chromatography on a column with Servacel-52
No. of Fractions |
Components of the Bee Venom |
VR, Ml |
M M, Kda |
1 |
Glycoprotein |
150 |
22 |
2 |
Phospholipasа A2 |
170 |
14.6 |
3 |
Mellitine |
180 |
12 |
Table 3 Data for separation of bee venom components by gel chromatography on a column with Sephadex G-75

From the above and on the basis of our experimental data, the eluent was selected and optimal conditions for separation and fractionation of the venom proteins were graded by stepwise elution with a concentration gradient of 0.01-1M sodium chloride solution on a Servacel-52 servacel column. Based on the results obtained, we believe that the ion exchange chromatography method can be used to separate the biologically active components of the honey bee venom. Thus, biologically active components of the poison with molecular weights of 12,000, 14,600 and 22,000D were isolated and identified. It should be noted that bee venom is a source for the production of biologically active peptides: melitin, apamin, adolapin, MSD-peptide, secapine, tertapine and enzymes: phospholipase A2, hyaluronidase. Mellitin, phospholipase A2 and glycoprotein, which exhibit antibacterial, antiviral and anti-inflammatory properties, are of particular interest among the biologically active components of honey bee venom. These components of the honey bee venom are more preferable alternatives to existing diagnostic and therapeutic drugs, since they have a faster activation rate, a lower probability of developing resistance reactions in the body, and the possibility of their use in combination with other drugs and preparations. At the same time, being a natural source for many drugs, in the near future, in combination with other biologically active components of natural origin, can become the basis for the development and production of promising and effective tools for the treatment and diagnosis of human diseases.
None.
The authors declare(s) that there is no conflict of interest regarding the publication of this article.

©2017 Mammadova, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.