MOJ
eISSN: 2379-6162


Coronary artery fistula (CAF) is a rare congenital anomaly that can be associated with intracardiac shunts, endocarditis, myocardial infarction, or coronary aneurysms. Recent reports have emphasized the efficacy of early closure either by percutaneous transcatheter or open surgical techniques. The purpose of this article is to review a case of 43year-old male who presented for repair of a giant aneurysmal left main coronary artery fistula emptying into the right atrium. The giant aneurysm fistula dilatation was maximally measured to be 4.3cmx3.5cm. There was clear left pulmonary vein compression and the giant size raised the question of aneurysm. With its unpredictable nature and patient’s worsening symptoms and physical activity limitation, a direct ligation of fistulous tract was performed. Surgical management was safe treatment and efficacious.
Keywords: 3D echo, CT angiogram, left main coronary artery, fistula, Laplace’s law
CAFs, coronary artery fistulae; CTA, computed tomography angiogram; CHF, congestive heart failure; SVC, superior vena cava; LAD, left anterior descending; TTE, transthoracic echocardiogram; VTI, velocity time integral
Coronary artery fistulae (CAF) are either congenital or acquired coronary artery abnormalities, that have different anatomical appearance; with varying degree of shunting (Qp/Qs); and associated cardiac anomalies.1 Etiologies include high cardiac output state and congestive heart failure with shunting of blood into a cardiac chamber, great vessel, or other structures, bypassing the myocardial capillary network.2 If the fistula is large, the intracoronary diastolic perfusion pressure diminishes progressively.1,2 The coronary vessel usually attempts to compensate by progressive enlargement of the ostia and feeding artery. Nevertheless, myocardium beyond the site of the fistula's origin is at risk for ischemia, most frequently evident in association with increased myocardial oxygen demand during exercise or activity.3 With time, the coronary artery leading to the fistulous tract dilates progressively, that in turn, may progress to frank aneurysm formation, intimal ulceration, medial degeneration, intimal rupture, atherosclerotic deposition, calcification, side-branch obstruction, mural thrombosis, and, rarely, rupture.4 Reports suggested surgical repair or transcatheter embolization in symptomatic patients at risk for fistula aneurysmal rupture and/or obstruction of nearby anatomic structures from its mass effect.5,6
So far, at least four anatomical variants of a giant CAF have been described in literature. (1) A right coronary fistula that drained into the SVC; (2) a left coronary fistula that drained into a main pulmonary artery and several plexal vessels that transversed through the pulmonary trunk and toward the pericardial reflex. Under cardiopulmonary bypass, the fistulas and plexal vessels were successfully ligated without any injury to the native coronary circulation7 (3) a right coronary artery fistula to the right atrium or coronary sinus,8a common site for fistulas, which has been reported multiple times and (4) more recently a 57-year-old man with CAF and giant aneurysm draining into the main pulmonary artery.9 This patient was treated successfully with surgical removal of both fistula and aneurysm with concomitant coronary artery bypass grafting. We report a case of 43year-old African American male with congestive heart failure (CHF) symptoms who presented with a large coronary artery fistula and giant fistulous aneurysm draining not into superior vena cava (SVC) and pulmonary artery but directly into the right atrium and an additional small right coronary artery to the right atrium.
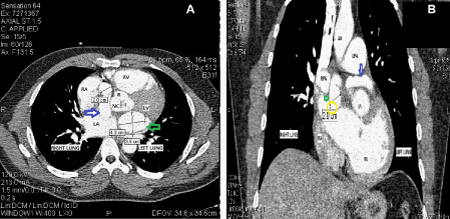
The Computed Tomography angiogram (CTA) of thorax showed a left main CAF with giant aneurysm dilatation measuring 4.3cmX3.5cm with a long fistula emptying into the right atrium (Figure 1A & Figure 1B). The size of the left main artery at origin of the left sinus of Valsalva and where it entered into the right atrium measured approximately 1cm. The aneurysmal fistula was seen to cause its mass effect on the left pulmonary vein and superior vena cava (SVC) near the atrial caval junction (Figure 2). Left main coronary artery gave rise to left anterior descending (LAD) and LCX, which maintained a normal course. RCA had a normal course and coronary sinus was normal in size as well. A transthoracic echocardiogram (TTE) showed normal systolic function with ejection fraction (EF) of 55% to 59%, moderately dilated right ventricle and left atrium, and a very large (3.2cm in diameter) dilated fistulous tract extending behind the aorta and superior to the left atrium emptying into the right atrium. Patient also underwent coronary angiogram which confirmed this fistulous tract and showed that he had no other flow-limited lesions of his LAD, circumflex, or RCA. The angiogram did reveal an additional small right coronary artery to the right atrial fistula. Considering his worsening CHF symptoms, potential risk of rupture of the giant aneurysmal fistula and compression of the pulmonary veins due to its mass effect, a decision was made to ligate this fistulous tract.
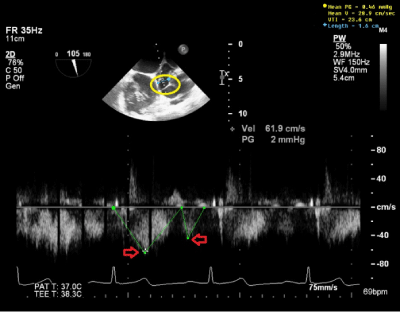
Intraoperative TEE ME SAX AV view with color Doppler shows a mosaic color pattern indicating blood flow in enlarged left main coronary artery and the fistula tract behind (Figure 3A). TEE ME Bicaval view with color Doppler indicates blood flow in distal left main coronary artery fistula and giant fistula aneurysm (Figure 3B) with different channeling tunnels seen per 3D (Figure 3C & Figure 3D), forming a complex network of trafficking communication between distal fistula tract, right atrium and giant aneurysmal CAF dilatation. The pulse wave Doppler through distal fistula tract allowed us to calculate velocity time integral (VTI) of 23.6cm (Figure 4). By equation: Volume=Area X VTI, the volume per heart beat shunted via fistula is calculated to be 47.2ml, using the measured cross sectional fistula diameter of 1.6cm (Area=2cm2), which equated to be 3.26L/min for the flow rate across the fistulous tract considering patient’s heart rate of 69beats/min. This shunting volume and flow rate indicated a significant L-R shunt, which correlated well with patient’s high CHF symptoms.

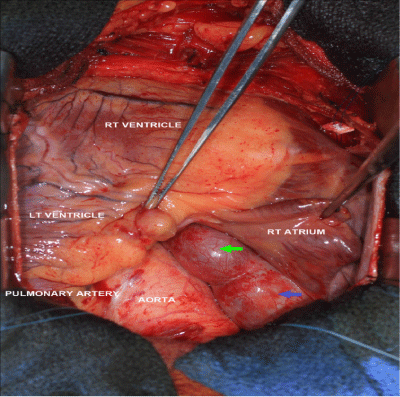
On cardiopulmonary bypass, the right atrial appendage was opened and a 2cm wide orifice was found between the SVC and the tricuspid valve in the medial wall of the right atrium. There was also a separate orifice (0.4cm in diameter) into the medial right atrium, which appeared to be the fistulous tract from the right coronary artery. These orifices were oversewn and the right atriotomy was closed. The fistula was observed to pass posteriorly behind the aorta and pulmonary artery. The left main coronary artery was opened longitudinally near the base of the left atrial appendage. The 1cm orifice to the fistula was oversewn with a running 5-0 Prolene suture in a double layer. The left main coronary artery was then closed using a running 5-0 Prolene suture in a double layer anticipating a closure of 7mm in diameter. Antegrade cardioplegic solution was administered to test homeostasis of the anastomosis. The total cross-clamp time was 59minutes and total cardiopulmonary bypass time was 83minutes. The patient was separated from cardiopulmonary bypass without difficulty. Patient had an uneventfully post-operative course. A CTA thorax follow-up study 3-weeks later showed the post-surgical change of a thrombosed fistula.
Our described case of a giant aneurysmal fistula draining into the right atrium was congenital and had grown with time. In this case, it caused a high cardiac output state with likelihood of coronary steal phenomenon. From the appearance on CT scan, we doubt a percutaneous approach would have been successful. The giant aneurysm fistula was seen to dilate to its maximum size at the end of systolic phase of cardiac cycle and soon after atrial kick during diastolic phase per echocardiography and pulse wave Doppler (Figure 4). The dynamic change of the aneurysmal size is due to continuing change of pressure gradients between fistula tract and right atrium, between fistula tract and aneurysm, and during different phases of cardiac contraction and relaxation. Under LaPlace’s Law, which describes the relationship between the transmural pressure (P) and the tension (T), radius (R), and thickness (T) of the vessel wall (P=k x 2HT/R) where k is a constant number, this condition could dramatically increase the aneurysmal fistula’s tension as such even a slight impact from a coiling procedure might make it prone to rupture. If the fistula were to have a narrow origin and ending, percutaneous treatment with a coil or a vascular plug in both ends could have been attempted.
Currently, percutaneous treatment is often attempted as first choice therapy because it is less invasive and entails a shorter period of hospitalization. Surgery is usually reserved for cases with affected large branches during embolization of coils, those with multiple fistulae, or when the fistulous connection is narrow, restrictive and draining into a cardiac chamber.10,11 Previously our group had also demonstrated in a relatively large series of 30patients from a single centre spanning over 17years that therapeutic strategies were based on symptoms and shunt size. We reported that conservative management was an option with small shunts and mild or no symptoms. Patients with moderate/severe symptoms and/or large shunts were treated with either percutaneous embolization or surgical ligation. However, our work concluded that management of CAF was still controversial and treatment of adult asymptomatic patients with non-significant shunting remained still a matter of debate.12 In our present case, this patient has a rare large left main coronary artery fistula with such a giant aneurysmal dilatation, a condition making the embolization with coil very technically challenging with the potential fearful complication of rupture.
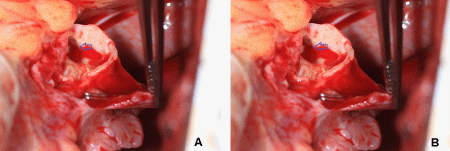
During surgery, we identified the drainage fistula from the right coronary artery that was very difficult to see on angiogram and not seen on echocardiography. This fistulous tract would have bled extensively if damaged or creating more shunting phenomenon affecting patient’s hemodynamic profile once the main fistula was closed. We used a surgical approach of ligation of the giant fistula with Prolene sutures in a double layer at both ends, one end from the fistula tract into the right atrium and the origin of the fistula tract (distance of 2cm measured per TEE). The closure was successfully tested by administering the antegrade cardioplegic solution, and TEE showed no flow in the large fistula body after the procedure per continuous wave Doppler and color Doppler (Figure 5A). TEE Live 3D Zoom from right atriotomy view also confirmed thrombosis in the giant fistula aneurysm (Figure 5B).
Our surgical approach as above, after thorough diagnosis work-up and careful search for any other abnormal congenital anomalies using CTA thorax, echocardiography and coronary angiogram was successful in treating a patient with rare large left main CAF and giant aneurysmal fistula dilatation draining into the right atrium. This technique seemed well applied.
We would like to thank Mr Richard Doerr (Department of Cardiac Surgery) and Dr Jacobo Vazquez (Department of Cardiology) for their help and referral.
The author declares no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.