MOJ
eISSN: 2374-6912


Research Article Volume 3 Issue 4
1Department of Biochemistry, JSS CACS (autonomous of University of Mysore), India
2Department of Biotechnology, JSS CACS, (autonomous of University of Mysore), India
3Department of Chemistry, JSS CACS, (autonomous of University of Mysore), India
Correspondence: Mukunda Chethankumar, Department of Biochemistry, JSS CACS (autonomous of University of Mysore), Mysuru City, Karnataka State, India, Tel +918884345956; +9108212548216, Fax +9108212548238
Received: April 06, 2016 | Published: May 20, 2016
Citation: Chethankumar M, Banu SH, Latha BV, et al. Studies on inhibition of H2O2 induced TGF– β1 expression in peripheral blood mononuclear cells by novel pyridine appended lutein derivative. MOJ Cell Sci Rep. 2016;3(4):96-101. DOI: 10.15406/mojcsr.2016.03.00061
Lutein a hydroxy carotenoid belonging to xanthophyll group having a hydroxyl group attached to either end of the molecule, making it react more strongly with singlet oxygen than other carotenoids. In the present study, Lutein was modified by appending pyridine moiety for increased antioxidant activity and its ability to inhibit H2O2 induced TGF-β1 expression in peripheral blood mononuclear cells was investigated. Lutein was treated with Isonicotinic acid (Pyridine-4-carboxylic acid) in the presence of DCC and DMF as solvent medium to obtain pyridine appended Lutein (2.28mg/ml). The pyridine appended Lutein was purified using silica gel-G (60–120mesh) column, further by HPLC and fractions were eluted at the rate of 1ml/min using water, acetonitrile, methanol and dichloromethane as mobile phase in the ratio 0.5:9.5:67.5:22.5. A major peak was obtained at 454nm with retention time of 1.60min. In dose dependent study, Pyridine appended Lutein at 0.42µmoles showed a maximum of 91.6% of DPPH radical scavenging activity in comparison to unmodified Lutein (80.76%). Unmodified Lutein showed maximum activity (86.18% at 1.25µmoles) with 2.97 folds higher than Pyridine appended Lutein. TGF–β1 expression in H2O2 (3mM) treated peripheral blood mononuclear cells was inhibited by 83.14% (152.1pg/ml) and 76.21% (214.62pg/ml) at 0.92µmoles of Pyridine appended Lutein and unmodified Lutein respectively compared to H2O2 treated cells (902.15pg/ml) without test samples. In conclusion, Pyridine appended Lutein showed higher DPPH radical scavenging activity and inhibited H2O2 induced TGF–β1 expression in peripheral blood mononuclear cells at low concentrations than unmodified Lutein.
Keywords: DPPH radical, isonicotinic acid, n,n’-dicyclohexylcarbodiimide, n,n-dimethyl formamide, lutein, TGF–β1
Carotenoids are fat soluble naturally occurring pigments that are synthesized by plants and microorganisms, serving a variety of roles in cell biology.1 Carotenoids are the pigments that involve in light harvesting to participate in energy transfer process in photosynthesis. They absorb light in the 400-500nm region of the visible spectrum and impart coloration of yellow, orange, red, and purple.2 They are widely distributed in nature including vegetables, fruits, insects, fishes, and birds. Animals are incapable of synthesizing carotenoids, hence should be obtained through diet.3 Among 700 carotenoids, 40 of them are present in the human dietary source and 20 carotenoids are present in the human blood and tissues.4,5 Lutein and Zeaxanthin belong to the xanthophyll family of carotenoids and are the two major components of the macular pigment of the retina.6 Lutein and Zeaxanthin differ from other carotenoids in that they each have two hydroxyl groups. The hydroxyl groups appear to control biological function of Lutein and Zeaxanthin.7 Lutein (β, ε-carotene-3, 3′-diol, C40H56O2) has no provitamin A activity and has two rings: one β-ring and another ε-ring hydroxylated at the 3 and 3′ position. It consists of eight isoprenoid units which appear as a conjugated double bond. The isoprenoid arrangement is reversed at the center of the molecule, so that the two central methyl groups are in α1,6-position and the remaining non-terminal methyl groups are in α1,5-position.8
The naturally occurring carotenoids are efficient antioxidants.9,10 Lutein is shown to possess pronounced free radical scavenging ability due to its polarity and number of conjugated double bonds,11 however chemical synthesis of their derivatives has proven to be better in their biological activity.12 Lutein-6H-1,2-oxazine along with 14-s-cis-5-nitrolutein is reported to exhibit better scavenging activity of peroxynitrite and nitrogen radicals.13 15-nitroastaxanthin, derivative of astaxanthin is reported to inhibit invitro Epstein-Barr virus early antigen activation and two-stage carcinogenesis in mouse skin papillomas. Similar results are reported for the 15-nitrolutein, a major reaction product of lutein with peroxynitrite.14 Partial synthesis of (3R, 6’R)-alpha-cryptoxanthin and (3R)-beta-cryptoxanthin from (3R, 3’R, 6’R)-lutein is reported with significant biological activity.15 ROS such as superoxide anion (O2•–), hydrogen peroxide ( H2O2), and hydroxyl radical (OH•), participate in cell signaling and/or injury.16 H2O2, in most biological contexts, is generally less reactive and long-lived than either superoxide anion or hydroxyl radical. H2O2 is shown to mediate intracellular signal transduction by its ability to oxidize cysteine residues in the catalytic domains of protein tyrosine phosphatises.17 ROS have been shown to mediate TGF–β induced cellular responses in various cells.18,19 TGF–β is a multifunctional cytokine that regulates variety of physiological process, including cell growth, differentiation, profibrotic gene expression, fibroblast proliferation and is a key regulator of fibrosis.20
The pyridine nucleus is an important heteroaromatic class of compounds with a wide range of activities. Nicotinic acid derivatives and its isomers have also been investigated as an agent for the prevention or delay of the onset of type 1 diabetes mellitus. They also have anti-bacterial, anti-oxidant, anti-inflammatory and anti-carcinogenic activities, and have putative activity against osteoarthritis.21 Isonicotinic acid also known as Pyridine-4-carboxylic acid is an organic compound with a carboxyl group on a pyridine ring. It is an isomer of nicotinic acid and the carboxyl group for isonicotinic acid is on the 4-position instead of the 3-position for nicotinic acid. It is non-hazardous and non-carcinogenic. It is stable at high temperature 3000C. The isonicotinic acid derivatives namely Rimifon and Marsilid have been used as a potential drug against pulmonary tuberculosis, and in the treatment of bone and joint tuberculosis.22,23 In the present study, Lutein was modified by appending pyridine moiety for increased antioxidant activity and its ability to inhibit H2O2 induced TGF–β1 expression in peripheral blood mononuclear cells was investigated. The peripheral blood mononuclear cells are used with the rationale that they are easy to achieve, relatively robust but heterogeneous cell types and limited replication. It is reported that these cells are found suitable for functional analysis of polymorphisms in the TGF–β signalling pathway.
Materials
Lutein was obtained from Sigma–Aldrich (St. Louis, MO). All the other chemicals and solvents used were of analytical grade from Merck Biosciences India Pvt. Ltd. Multispecies TGF–β1 ELISA kit (#KAC1688) was procured from Invitrogen Corporation, CA. RPMI– 1640 medium, fetal bovine serum (FBS), bovine serum albumin (BSA), trypsin–EDTA, Hank’s Balanced Salt Solution (HBSS), sodium bicarbonate, gentamycin, phosphate- buffered saline (PBS), Triton X–100, penicillin and streptomycin was procured from Sigma–Aldrich (St. Louis, MO).
Human peripheral lymphocyte isolation and culture
The peripheral lymphocytes were isolated from 15ml of freshly drawn venous blood from healthy male donors aged between 25-30years. Blood was collected in anticoagulant Acid Citrate Dextrose (ACD: 85mM citric acid, 71mM trisodium citrate, 165mM D-glucose) in the ratio of 5:1. Four volumes of hemolysing buffer (0.85% NH4Cl in 10mM Tris buffer, pH7.4) was added, mixed well and incubated at 40C for 30min. Then the cells were centrifuged at 1200rpm for 12min, the supernatant was discarded, pellet was washed again in 5ml of hemolysing buffer and the cell pellet was washed thrice with 10ml of HBSS (137mM NaCl, 5mM KCl, 0.8mM MgSO4, 5mM D-glucose in 8.5mM phosphate buffer pH7.4) and suspended in the same buffer solution. Cells were suspended in RPMI-1640 media supplemented with 12% fetal calf serum supplemented with glutamine. Temperature was maintained at 370°C in a humidified 5% CO2 incubator.24 The medium was changed at intervals of 24hrs.
Cell viability assay
The cells are removed from the medium replaced with 100µL of fresh culture medium. 10µL of the 12mM MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) stock solution was added to each well. A negative control of 10µL of the MTT stock solution added to 100µL of medium alone was used. The cells were incubated at 37°C for 4hrs. 100µL of the SDS-HCl solution was added to each well and mixed thoroughly. The microplate was incubated at 37°C for 4hrs in a humidified chamber.25 Each sample was mixed and the absorbance was read at 570nm.
Derivatization of lutein by condensation reaction
Lutein Derivatization by condensation reaction using pyridine-4-carboxylic acid was carried out with modifications the method reported earlier.26 21mg of pyridine-4-carboxylic acid was treated with 33mg of DCC in the presence of 25ml of DMF as a solvent medium for reaction to occur in a round bottom flask. The mixture was stirred for 15min to form an intermediate. To this intermediate, 100mg of Lutein was added, stirred for 24hrs to obtain Pyridine appended Lutein along with a minor product dicyclohexyl uridine (DHU). The Pyridine appended Lutein was transferred to the separating funnel followed by the addition of chloroform:water (1:1). After vigorous shaking Pyridine appended Lutein present in the chloroform phase was separated and stored in a dark brown bottle at −200C for further studies.
Purification pyridine appended lutein using silica gel chromatography and HPLC
Pyridine appended Lutein was purified using 10% silica gel 60−120 mesh suspended in n-hexane. The slurry was packed onto 25X1.2cm column. 1mg of Pyridine appended Lutein in hexane was loaded onto the column and eluted with n-hexane:ethyl acetate in the ratio of 70:30(v/v). The fractions were collected at a flow rate of 2ml/5min. The fractions showing maximum absorbance at 454nm were pooled and further concentrated by Rotavapor.27 The HPLC purification was done using Agilent 1260 Infinity Quaternary LC System composed of G1311B/C Quaternary Pump, G1329B Autosampler, G1330B Thermostat and G4212B DAD. The HPLC system was equipped with Eclipse plus C18 column (4.6X150mm I.D., 5µm particle). The analysis of the chromatographic data was carried out on Open lab CDS ChemStation software (A.01.05). The fractions were eluted at the rate of 1ml/min using water, acetonitrile, methanol and dichloromethane as mobile phase in the ratio 0.5:9.5:67.5:22.5.
Analysis of pyridine appended lutein by liquid chromatography mass spectrometery
The Pyridine Appended Lutein was analyzed by LCMS. MS was carried out in the positive ion measurement mode with a detection voltage of 1.6kV, with the flow rate of the nebulizer gas was 2.5ml/min. Full scan spectra were obtained by scanning masses between m/z 100 and 700.
Scavenging activity against DPPH radical
The effect of Pyridine appended Lutein on the DPPH radical was estimated according to the method of reported earlier.28 100mM Tris-HCl buffer (500µl, pH 7.4) with 150µl of the DPPH in ethanol was mixed to a final concentration of 250µM with or without aliquots of Pyridine appended Lutein, unmodified Lutein and BHA (0 to 1.70µmoles in 30µl each). The mixture was shaken vigorously and left to stand at room temperature for 20min in the dark. The absorbance at 517nm of the reaction solution was measured spectrophometrically. The DPPH decolourization of the sample was calculated according to the following equation as, % decolourization=(1―Absorbance of sample/Absorbance of control) X 100.
Quantitative determination of TGF−β1 using ELISA
TGF−β1 was quantified using multispecies TGF−β1 kit as per the manufacturer’s instruction. The Invitrogen Multispecies TGF−β1 kit is a solid phase sandwich ELISA. A monoclonal antibody specific for TGF−β1 coated onto the wells of the microtiter strips were used. Samples, including standards of known TGF−β1 content, control specimens, and unknowns (3mM H2O2) treated peripheral blood mononuclear cells with or without 0 to 2.70µmoles of Pyridine appended Lutein and unmodified Lutein each, were pipetted into these wells, and followed by the addition of a biotinylated secondary antibody. After removing excess detection antibody, streptavidin−peroxidase was added. After a second incubation and washing to remove the entire unbound enzyme, a substrate solution was added, which was acted upon by the bound enzyme to produce color. The intensity of the colored product measured at 450nm was directly proportional to the concentration of TGF−β1 present in the sample.
Analysis of Variance (ANOVA) test, followed by individual comparison by Student’s ‘t’–test, for the determination of level of significance among the mean ± SEM, in various groups were performed.
Derivatization of Lutein by condensation reaction
In the present investigation, Lutein was derivatized by condensation reaction using pyridine-4-carboxylic acid. 21mg of pyridine-4-carboxylic acid when treated with 33mg of DCC in the presence of 25ml of DMF as a solvent medium yielded 2.28mg/ml of pyridine appended Lutein along with a minor product dicyclohexyl uridine (DHU). The pyridine appended Lutein showed maximum absorbance at 454nm.
Purification Pyridine appended Lutein using silica gel chromatography and HPLC
Purification using silica gel (60-120 mesh) showed two peaks, with major peak at 454nm and minor peak at 477nm. The fractions which showed maximum absorbance at 454 nm were pooled and concentrated using Rotavapor. The dried pyridine appended Lutein was suspended in methanol and further purified by HPLC and fractions were eluted at the rate of 1ml/min using water, acetonitrile, methanol and dichloromethane as mobile phase in the ratio 0.5:9.5:67.5:22.5. A major peak was obtained at 454nm with retention time of 1.60min. Unmodified Lutein showed single major peak at 444nm with retention time of 1.82min (Figure 1A and B).

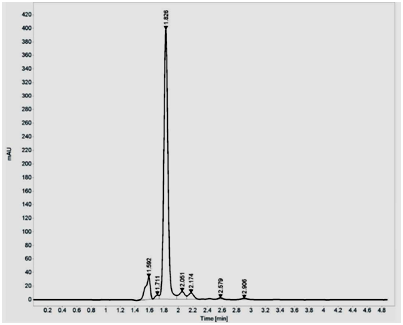
Figure 1 HPLC purification of pyridine appended Lutein (A) and unmodified Lutein (B) on Agilent 1260 Infinity Quaternary LC System composed of G1311B/C Quaternary Pump, G1329B Autosampler, G1330B Thermostat, G4212B DAD equipped with Eclipse plus C18 column (4.6X150mm I.D., 5µm particle). The analysis of the chromatographic data was carried out on Open lab CDS ChemStation software (A.01.05). The fractions were eluted at the rate of 1ml/min using water, acetonitrile, methanol and dichloromethane as mobile phase in the ratio 0.5:9.5:67.5:22.5.
Analysis of Pyridine Appended Lutein by Liquid Chromatography Mass Spectrometery
The Pyridine Appended Lutein analyzed by LCMS gave an molecular mass of 650Da confirming the compound as parent molecule Lutein appended with pyridine moiety (Figure 2).
Scavenging activity against DPPH radical
Pyridine appended Lutein at 0.42µmoles showed 91.60% of DPPH radical scavenging activity in comparison to unmodified Lutein (80.76%), whereas, butylated hydroxy anisole (BHA) scavenged DPPH radicals by 88%. Further, increasing the concentrations of unmodified Lutein and BHA from 0.42µmoles up to 1.70µmoles showed moderate increase in DPPH scavenging activity to 86.18% and 89.4% respectively. There was no significant change in the DPPH scavenging activity by pyridine appended Lutein by increasing the concentration to 1.70µmoles. The unmodified Lutein and BHA showed maximum scavenging activity of 86.18% and 89.4% when used at concentrations 2.97 folds higher than pyridine appended Lutein (Figure 3). According to the statistical analysis the values were considered significant at p<0.05 from 0.42µmoles.

Figure 2 Analysis of Pyridine Appended Lutein by Liquid Chromatography Mass Spectrometery.
The Pyridine Appended Lutein was analyzed by LCMS. MS was carried out in the positive ion measurement mode with a detection voltage of 1.6 kV, with the flow rate of the nebulizer gas was 2.5 ml/min. Full scan spectra were obtained by scanning masses between m/z 100 and 700.

Figure 3 Scavenging activities against DPPH radical. 100mM Tris-HCl buffer (500µl, pH 7.4) and 150µl of the DPPH mixed in ethanol to a final concentration of 250µM with or without pyridine appended Lutein, unmodified Lutein and BHA as mentioned in methods. The DPPH decolourization of the sample was calculated according to the following equation as, % decolourization= (1―Absorbance of sample/Absorbance of control) X 100. The assay was performed in triplicates and the values are expressed as Mean±SD.
Quantitative determination of TGF−β1 using ELISA
Effect of pyridine appended Lutein on TGF−β1 expression in H2O2 treated peripheral blood mononuclear cells was examined. After pre-treatment with different concentrations of pyridine appended Lutein and unmodified Lutein (0−2.70µmoles) for 30min, the cells were cultured with H2O2 (3mM) for 48hrs. Pyridine appended Lutein and unmodified Lutein at 0.92µmoles inhibited TGF−β1 expression in H2O2 (3mM) treated peripheral blood mononuclear cells by 83.14% (152.1pg/ml) and 76.21% (214.62pg/ml) respectively compared to H2O2 treated cells (902.15pg/ml) without test samples (Figure 4). Further increase in the concentrations of pyridine appended Lutein from 0.92µmoles to 2.70µmoles did not show significant inhibition of TGF−β1 expression, whereas unmodified Lutein showed 79.26% of inhibition. The cell viability was always above 90% in the assay performed. The pyridine appended Lutein and unmodified Lutein was not cytotoxic even at concentrations at 5.0µmoles (Table 1). According to the statistical analysis the values were considered significant at p<0.05 from 0.92µmoles.
Concentration in µmoles |
% of Cells Viable |
|
|---|---|---|
Pyridine appended Lutein |
Unmodified Lutein |
|
0.5 |
96±5.0 |
95±6.0 |
2 |
95±6.0 |
94±4.0 |
5 |
96±4.7 |
96±5.2 |
Table 1 The cell viability assay
10µL of the 12 mM MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) stock solution was added to each well. A negative control of 10µL of the MTT stock solution added to 100µL of medium alone was used. The cells were incubated at 37°C for 4hrs. 100µL of the SDS-HCl solution was added to each well and mixed thoroughly. The microplate was incubated at 37°C for 4hrs in a humidified chamber. Each sample was mixed and the absorbance was read at 570nm. The assay was performed in triplicates and the values are expressed as Mean±SD

Figure 4 Quantitative determination of TGF−β1 using ELISA with samples, including standards of known TGF−β1 content, control specimens, and unknowns (3mM H2O2) treated peripheral blood mononuclear cells with or without Pyridine appended Lutein and unmodified Lutein. The intensity of the colored product measured at 450nm was directly proportional to the concentration of TGF−β1 present in the sample. The assay was performed in triplicates and the values are expressed as Mean±SD.
This study demonstrated the synthesis of pyridine appended Lutein by treating Lutein with Isonicotinic acid (Pyridine-4-carboxylic acid) in the presence of DCC and DMF, further examined the effect of pyridine appended Lutein on DPPH radical scavenging activity and TGF– β expression in H2O2 treated peripheral blood mononuclear cells.
The xanthophyll Lutein is not endogenously synthesized by the human body and tissue levels therefore depend on dietary intake.29 Lutein is an antioxidant which is believed to be an essential nutrient for normal vision.30 Studies have also indicated that Lutein improves heart health, protects skin against UV damage, reduces oxidative stress, and possesses anti-inflammatory and anti-cancer properties.31 Lutein is hydrophobic in nature since the presence of long carbon chain and therefore its uptake is bound to fats. Better absorption could be achieved by enhancing water solubility. This may increase the antioxidant properties as well. Several studies are reported on the introduction of novel reactions to Lutein chemistry.12,13 In recent years, attempts have been made to increase the hydrophilicity of Lutein due to its potential applications in the medicine and food industries. Pyridine is a heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one CH group replaced by a nitrogen atom. It is used as a precursor to agrochemicals and pharmaceuticals and is also an important solvent and reagent. Pyridine is found to be a very versatile nucleus in the pharmaceutical field. The derivatives are very much used as anticancer, antimicrobial, antiviral, antidiabetic & antithrombic agents etc.32 In the present investigation, the pyridine appended Lutein showed a maximum absorbance at 454nm compared to unmodified Lutein (444nm). According to Fieser Rule, the absorption maxima of the plant pigments increases with the number of newly added conjugated double bonds, alkyl substituents, endocyclic and exocyclic double bonds.33 Hence the increase in the maximum absorption for pyridine appended Lutein might be due to the additional pyridine group conjugated to the Lutein.
Lutein is shown to possess pronounced free radical scavenging ability due to its polarity and number of conjugated double bonds,11 was appended with pyridine moiety. Pyridine derivatives are also shown to possess significant DPPH radical scavenging activity.34,35 Thus chemical synthesis of such derivatives has shown to possess better biological activity. The presence of the pyridine moiety increased the DPPH radical scavenging activity probably due to enhanced proton donor capacity. On the other hand, Pyridine ring is known to increase the lipophilic character of the molecule Lutein, which facilitates the crossing through the biological membranes, thus increasing the availability of the Lutein for improved antioxidant activity. Alkyl groups at positions 2 and 4 of a pyridine ring are more reactive and carbanions can be formed readily at alkyl carbons attached at the 2- and 4-positions. This increased chemical reactivity is used to form the pyridine appended derivative. Chemical reactivity of pyridines is a function of ring aromaticity, presence of a basic ring nitrogen atom, π-deficient character of the ring, large permanent dipole moment, easy polarizability of the π-electrons, activation of functional groups attached to the ring, and presence of electron-deficient carbon atom centres at the α- and γ-positions. Depending on the conditions of the chemical transformation, one or more of these factors can give rise to the observed chemistry. The Ring-atomic centres can undergo attack by electrophiles, easily at the ring nitrogen and less easily at ring carbons. Nucleophile attack is also possible at ring carbons or hydrogens. The strong biological activity of pyridine appended Lutein could be due to the fact that the molecule makes the electrons more delocalized.36 ROS are excessively produced in several disease states, and their injurious effects may contribute to the pathogenesis of many diseases. The relationship between increased ROS synthesis and the cellular functional and morphological changes has been explored using various experimental approaches. ROS vary in their inherent reactivates, stability, chemistry, and diffusibility. H2O2, in most biological contexts, is generally less reactive and more long−lived than either superoxide anion or hydroxy radical. Moreover, H2O2 is lipid−soluble and can diffuse across biological membranes. Most studies of H2O2 have focused on its role in mediating intracellular signal transduction by its ability to oxidize cysteine residues in the catalytic domains of protein tyrosine phosphatases [37,38]. Biological effects of extracellular H2O2 have been primarily studied by adding exogenous H2O2 to target cells.39
TGF−β is a potent fibrogenic cytokine and found to induce oxidative stress. Oxygen derivatives, acting as secondary intracellular messengers, have been shown to activate transcription factors, such as nuclear factor−κB (NF−κB) and activated protein−1 (AP−1). ROS may stimulate the transcription and production of TGF− β. The role of TGF−β in inducing extracellular matrix production is widely reported. TGF−β levels are known to be significantly elevated in H2O2 treated wounds thus indicating the involvement of H2O2 in activating TGF−β. There are several approaches reported to neutralize TGF−β activity. These include use of proteoglycan decorin, soluble TGF−β type II receptors and gene therapy approaches.40 From the study it is understood that pyridine appended Lutein could serve as much better approach for neutralizing TGF−β1 activity at much lesser concentrations than unmodified Lutein or Pyridine alone.
In conclusion, the present study showed that the newly synthesized pyridine appended Lutein showed remarkable increase in DPPH radical scavenging activity at very low concentration than unmodified Lutein and further significantly inhibited H2O2 induced TGF–β1 expression in peripheral blood mononuclear cells.
The study was funded by Science and Engineering Research Board (SERB) of Dept. of Science and Technology (DST), Govt. of India under Fast Track Scheme (No. SR/FT/LS-159/2010).
The author declares no conflict of interest.

©2016 Chethankumar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.