MOJ
eISSN: 2573-2951


This study explores the relationship between theoretical and experimental prediction of hot melt extrudate miscibility and subsequent performance on storage using a relevant model system. More specifically, an investigation was conducted into the miscibility and separation of ibuprofen within hot melt extruded matrices of Eudragit RS PO intended for transdermal delivery. Theoretical miscibility was assessed via melting point depression and solubility parameter calculation, while experimental values were obtained using thermal, imaging and spectroscopic analysis. The addition of ibuprofen was found to assist processing in terms of reducing torque via plasticization effects. The incorporation of ibuprofen in the polymeric matrices was assessed as a function of time, both in the dry state and on exposure to ambient relative humidity. A good correlation was found between the theoretical and practical estimates of solubility, with high drug loadings (30‒35% w/w) achieved which remained stable over a month at low humidity storage, despite the low Tg of the systems. The stability was reduced for systems stored at 60% RH/25°C despite the water uptake being relatively low, indicating a marked dependence of miscibility on water content. Fragility calculations were also performed on the mixed systems, indicating high strength and stability. Overall, the study has demonstrated that ibuprofen incorporation in Eudragit RS PO may be estimated both theoretically and practically, with stable systems noted when stored under dry conditions.
Keywords: extrusion, solid dispersion, amorphous, solubility, polymeric drug delivery system, physical characterization, mathematical model, physical stability
HME, hot melt extrusion; NSAIDs, non‒steroidal anti‒inflammatory agents; GIT, gastrointestinal tract; TDDS, transdermal drug delivery system; MTDSC, modulated temperature dsc; RCS, refrigerated cooling system; ATR‒FTIR, attenuated total reflectance‒fourier transform infrared; PXRD, powder x‒ray diffraction; SEM, scanning electron microscopy; TGA, thermo gravimetric analysis.
Hot melt extrusion (HME) technology has been widely utilized for several applications within the pharmaceutical field, particularly in the context of formulating poorly water soluble drugs for oral delivery.1-3 The approach may also be used to prepare topical and Transdermal systems, a significant advantage being the negation of the need to use solvents.4-6 Nevertheless, the requirements for such systems are extremely similar to those of oral systems in that there is a need for the drug to remain in a stable form throughout the shelf life of the product. If the drug is presented as a molecular dispersion, this carries particular challenges in terms of stability and phase separation as the drug returns to the crystalline state on storage. While theoretical and practical approaches to predicting and measuring miscibility are available, here we examine the utility of these methods in predicting storage stability using a relevant model system. More specifically, we examine the question as to whether predictive miscibility measurements may be used as a measure of subsequent processing and storage stability. While predictive approaches have been examined in a number of thorough studies, the link between prediction, miscibility and storage behaviour is not well established.
In this work ibuprofen in Eudragit RS PO was formulated as hot melt extruded films. Ibuprofen (2-(4-isobutyl-phenyl) propionic acid) has a molecular weight of 206.28 and is hydrophobic in nature. Despite being categorized as one of the safest non-steroidal anti-inflammatory agents (NSAIDs) available,7 ibuprofen may produce gastrointestinal tract (GIT) adverse effects if administered orally. Therefore, there has been great interest in developing Transdermal ibuprofen systems for both local and systemic delivery.8-13 Eudragit RS PO is a well-recognized polymethacrylate polymer that may be used for pharmaceutical applications including HME.14-20 The material is a cationic copolymer synthesized from acrylic acid and methacrylic acid esters with 5% of functional quaternary ammonium groups.21 Eudragit RS PO is water insoluble,22 yet is able to swell and become permeable to solutes due to the presence of the ionized hydrophilic ammonium groups;23 it has been previously used in Transdermal drug delivery24-26 and is considered to be biocompatible and biologically safe.27
HME presents a means of preparing dosage forms in a scalable manner without the need for solvent use and removal; hence it represents a potentially valuable means of preparing film-based systems for Transdermal use.5-7,28,29 However, one must consider both the process ability of the systems, particularly in terms of the use of elevated temperatures and mechanical force, plus the form and stability of the product, particularly in terms of miscibility. These two considerations may be mutually dependent as the drug may plasticize the polymer matrix, thereby aiding processing, but by the same token the robust conditions used may lead to an inflated incorporation ratio, resulting in later phase separation.30 Furthermore, characterisation of drug solubility within a Transdermal drug delivery system (TDDS) is crucial for performance prediction, since flux is a function of activity, which may in turn be determined by equilibrium solubility within the matrix.31,32 Overall, therefore, effective prediction and characterisation of incorporation and associated stability is essential for effective product development.
Our hypothesis is that theoretical and practical approaches will allow correlation not only with each other but also to the storage stability of hot melt extruded systems. We have deliberately chosen a low Tg drug to study this approach as such materials are intrinsically more difficult to formulate and hence represent a raised level of challenge to achieve stability. In this context, therefore, recognized theoretical and experimental approaches were used to predict miscibility between Eudragit RS PO and ibuprofen. These data were then used to explore the correlation between predicted miscibility and corresponding extrudate miscibility and stability under a range of conditions in order to assess the utility of the predictive approaches for product development.
Materials
Crystalline ibuprofen (a racemic mixture of (+)S- and (-)R-enantiomers) was kindly donated by BASF (Ludwigshafen, Germany) and Eudragit RS PO was kindly donated by Evonik Röhm GmbH (Darmstadt, Germany).
Methods
Preparation of ibuprofen-Eudragit RS PO extrudates: Known amounts of crystalline ibuprofen were physically mixed with Eudragit RS PO using a pestle and mortar. Physical mixes of ibuprofen-Eudragit RS PO were compounded in a co-rotating twin screw extruder (Haake Minilab II Micro Compounder, Thermo Fisher Scientific, USA ) using a temperature of 100 ˚C from the feed to the die (approximate opening of 1 mm) and a screw speed of 100 rpm for four minutes. The resulting extrudates were prepared at different drug loadings until opaque extrudate was produced (1-40% (w/w) ibuprofen loading). Physical mixtures were also evaluated at loadings between 1-90% (w/w).
Physical characterisation: Conventional DSC and modulated temperature DSC (MTDSC) measurements were performed using a Q1000 DSC (TA Instruments, USA) equipped with a refrigerated cooling system (RCS). Temperature calibration was performed using indium, benzoic acid and n-octadecane, while heat capacity calibration was performed using aluminium oxide. Data were analysed using TA Universal Analysis Advantage Software v5.5.3. Nitrogen was used as the purge gas through the DSC cell at a flow rate of 50ml/min. TA instruments standard pans were used for all calorimetric studies and the mass of each empty sample pan was matched to the mass of the empty reference pan within ±0.05mg; all measurements were performed in triplicate.
Conventional DSC was used for several aspects of the study. Amorphous ibuprofen was obtained and studied via heating in open standard aluminium pan at 10 °C/min to 100 °C, then cooled to -70 °C at 20 °C/min followed by heating at 10 °C/min to 100 °C. For melting point depression studies, physical mixes were studied using samples of approximately 10 mg at different ibuprofen loadings using 1 °C/min heating rate. Samples were equilibrated at -70 ˚C for 5 minutes followed by heating to 100˚C. For fragility measurements, fresh extruded samples (4-6mg) of Eudragit RS PO loaded with ibuprofen 10-30% (w/w) were subjected to three steps using 10 °C/min as the reheating rate. The first step was equilibration at 70 °C, isothermal heating for 5 minutes and then a second step of cooling to -20 °C at different rates for each cycle: 2, 5, 10 and 20 °C/min. In each individual cooling rate experiment, isothermal condition was maintained for 5 minutes at -20 °C. The third step involved reheating to 70 °C (10 °C/min). For the MTDSC experiments, amplitude of ±0.265 °C, a period of 100 seconds and an underlying heating rate of 1 °C/min were used. The samples (4-6mg) in standard aluminium panswere equilibrated at -70 °C for 5 minutes followed by heating to 100 °C. The glass transition temperatures (Tg) were determined from reversing heat flow signals as the mid-points of not less than three measurements.
Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) spectrometry (Bruker IFS 66/S, Burker optics equipped with a Golden Gate ATR accessory from Specac Limited) was used to obtain spectra of the physical mixes and extrudates over a range of 4000-550 cm-1, 32 scans at a resolution of 4 cm-1. Omnic software (version 6.1a, Thermo Scientific, USA) was used to analyse results and perform comparison.
Powder X-ray diffraction (PXRD) was performed using an X-ray powder diffractometer (ARL Xtra, Thermo Fisher Scientific) equipped with X-ray tube (Copper, wavelength of 1.540562 Angstrom) over the 2Ө range from 2 to 60 degrees, scanning speed of 1.2°/min (step: 0.01 degree, 0.5 sec/step) using a 40 kV generator and a 20 mA stream.
Scanning electron microscopy (SEM) was used to examine morphological features of the surfaces and cross sections of the extruded formulations. The samples were mounted onto stubs using double-sided tape and were gold coated, in order to increase conductivity of the electron beam, by a Polaron SC7640 sputter gold coater manufactured by Quorum Technologies (UK), using a plasma current of 20 mA, Voltage: 2.1 kV for 30 seconds. Images were obtained using scanning electron microscope (JSM 5900LV; JEOL, Japan) fitted with a tungsten (W) filament, acceleration voltage of 20 kV and 10 mm working distance.
Storage studies of extruded ibuprofen-Eudragit RS PO films with drug loadings of 1-40% (w/w) were performed under different humidity levels for one month. The extruded films were stored at 0% RH and 60%RH, both at room temperature (24±1 °C). Airtight jars were used as humidity chambers with phosphorus pent oxide to achieve dryness (0% RH), and saturated salt solutions of sodium bromide in distilled water to control relative humidity to a level of ~60% RH at room temperature. Water contents were measured using thermo gravimetric analysis (TGA; TA Instruments TGA Q5000 IR) using a heating rate of 10 °C/min from 30 °C to 250 °C. All TGA runs were performed in open aluminium pans with a dry nitrogen gas purged at flow rates of 25 ml min-1 and 10 ml min-1 through the furnace and TGA head, respectively.
Theoretical assessment of the miscibility of ibuprofen-Eudragit RS PO hot melt extruded systems
Miscibility behaviour of the drug with the polymer and possible solubility in solid dispersions can be estimated using solubility parameters, which are related to the cohesive energy density of a molecule.33 The solubility parameters of the ibuprofen and Eudragit RS PO were calculated using the Hansen (partial) solubility parameter model; this approach has been shown to be of use in predicting solubility of pharmaceutical and other materials.34-36 In this model, the total energy is divided into individual components, namely, dispersion, polar and hydrogen bonding. This can be estimated through calculation of the total solubility parameter (δt) as follows:21
...Eq. (1)
Where δd is the dispersion, δp is the polar and δh is the hydrogen-bonding solubility parameter. These solubility parameters can be predicted using group contributions derived from the structures of the two materials.38 Accordingly the overall value of solubility parameters (δt) for ibuprofen and Eudragit RS PO were calculated based on the group contributions of their structural components; these values were found equal to 19.26 and 17.45 (J/cm3)1/2 for ibuprofen and Eudragit RS PO polymer, respectively, in agreement with the values obtained by Wu and McGinity39 for ibuprofen and Saerens et al.,40 Wang et al.41 for Eudragit RS PO. Therefore a good miscibility between these two components is predicted, as the difference (1.81 (J/cm3)1/2) is substantially less than the suggested maximum for miscibility of ±6.3 (J/cm3)1/2.42
Melting point depression has also been demonstrated as a feasible approach to predict miscibility of pharmaceutical materials.43-46 Marsac et al.44 suggested application of the Flory-Huggins theory to estimate drug-polymer miscibility through determination of the interaction parameter χ. In this work, χ was determined experimentally from melting point depression data using DSC and the following equation:
...Eq. (2)
where TMmix is the melting temperature of the drug in the presence of the polymer, TMpure is the melting temperature of the drug in the absence of the polymer, ΔHfus is the heat of fusion of the pure drug, and m is the ratio of the volume of the polymer to that of the lattice site (defined here by the volume of the drug), Φdrug is the volume fraction of the drug, Φpolymer is the volume fraction of the polymer, χ is the Flory-Huggins interaction parameter and R is the universal gas constant. This equation provides an estimate of the interaction parameter at temperatures close to the melting point of the drug and requires that there is enough physical interaction between the drug and the polymer for melting point depression to be manifested, in addition to thermal stability over the temperature range of interest.
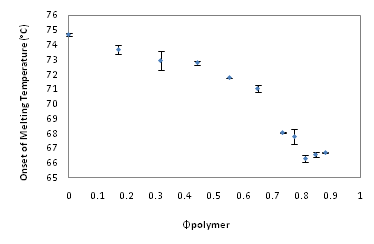
The melting point depression of crystalline ibuprofen blended with amorphous Eudragit RS PO was observed with increased volume fraction of this polymer in physical mixes, as shown in Figure 1. The temperature reduction is predicted to be caused by the decrease of the chemical potential of the drug by the addition of a miscible diluent.45

The resultant depressed melting temperatures can be then analysed using Equation (2) and plotted as shown in Figure 2 to estimate Flory-Huggins interaction parameter χ based on the data from the volume fraction of the polymer that yielded a linear relationship (R2 value of 0.9794). The value so obtained was -0.6559; this negative value predicts miscibility.44 Furthermore, this interaction parameter was used to estimate the drug solubility limit based on the following equation:
...Eq. (3)
Where γ (drug) is the activity coefficient of the drug in the polymer at solubility limits, m is the ratio of the volume of the material to the lattice site, x is the drug solubility mole fraction, χ is the Flory-Huggins interaction parameter and Φ is the volume fraction of the material.44 With the assumption that the activity coefficient is the same at the solubility limit of the drug in the polymer, the solubility limit of ibuprofen in Eudragit RS PO was calculated and was found to be circa 46.8% (w/w).
Practical assessment of the miscibility of ibuprofen-Eudragit RS PO hot melt extruded systems
Translucent hot melt extrudates were produced between 1-35% (w/w) drug loading, although opacity was noted at 40% (w/w) drug loading (Figure 3a). It was also observed that torque values inside the extruder decreased as a function of increased drug loading as illustrated in (Figure 3b). This parameter is directly proportional to the viscosity of the molten material, thus implying that ibuprofen decreased the melt viscosity via its role as a plasticizer.47-48
The extrudates were characterised by single glass transition temperatures over a concentration range of 1-35% (w/w) drug loading, as shown in the MTDSC reversing heat flow signal (Figure 4); the presence of single glass transition temperature implies single phase system with the drug molecularly dispersed in the interstitial spaces between the amorphous polymer chains.49 The glass transition temperature of the extruded systems decreased with increasing drug loading, again indicating that ibuprofen is acting as a plasticizer for Eudragit RS PO. In contrast, hot melt extruded systems at 40% (w/w) drug loading showed an endothermic peak related to the melting of the surplus ibuprofen with a depressed melting temperature of 66.9±0.4 °C (n=3), while also showing a glass transition temperature at 23.2±1.9 °C, indicating the plasticized drug-polymer glassy phase. An additional lower-temperature endotherm was also observed with a peak temperature at 42.9±0.9 °C. This value could reasonably correlate with the reported melting temperature of the eutectic of the ibuprofen enantiomers R(-) or S(+) between 46-52 °C [50-52].The observations presented here are therefore consistent with the process resulting in enantiomeric separation of ibuprofen upon melting,49 and then recrystallization (transition exotherm) as a racemic mixture.53,54

The compositional variation of the glass transition temperature of the previously described extruded systems of ibuprofen and Eudragit RS PO was further studied using the Gordon-Taylor (G-T) equation. This equation is used most often in the amorphous pharmaceutical materials due to its simplicity and reliability.55 Assuming ideal behavior of no specific interactions and perfect volume additivity during mixing of the studied mixtures, this equation can be used to predict glass transition temperature of these mixes with small positive or negative deviations.
This equation (G-T) can be represented as follows:56
...Eq. (4)
In which Tg1 and Tg2 are the glass transition temperatures of amorphous ibuprofen (-43.4±0.2 °C) and Eudragit RS PO (53.3±0.5 °C), respectively, whereby Tgmix is the predicted glass transition temperature of their mixture, w1 and w2 are the weight fractions of component 1 (ibuprofen) and 2 (Eudragit RS PO) in the mixture. K is a sample-specific parameter, which can be estimated using the popular Simha-Boyer rule:57
...Eq. (5)
Where ρ1 is the density of component 1 and ρ2 is the density of component 2. The densities are 1.05g/cm358,59 for ibuprofen and 0.57 g/cm3 for Eudragit RS PO. Using Equations 4 and 5, the predicted theoretical values of glass transition temperatures of the binary extruded systems of ibuprofen (component 1) and Eudragit RS PO (component 2) with varying compositions is shown in Figure 5. As can be seen from this Figure, the experimental data deviate negatively from the theoretical values obtained from the G-T equation up to 35% (w/w) drug concentration. However, at 40% (w/w) ibuprofen loading an increase of the glass transition temperature was observed which almost meets with the predicted or theoretical value derived from the G-T model. It is therefore suggested that a degree of super saturation has occurred, which is negated when the dissolved excess drug crystallizes out, and hence less of plasticizing effect.
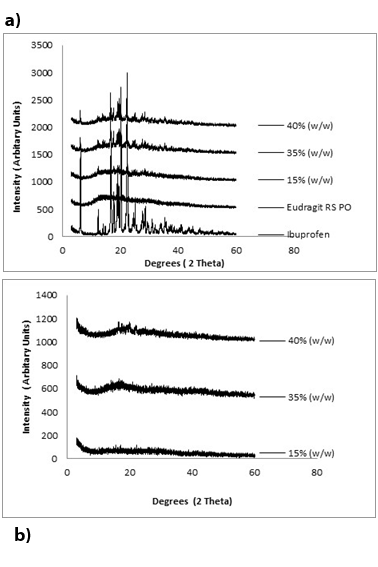
(Figure 6a) and (Figure 6b) show an overlay of the X-ray powder diffraction profiles (diffract grams) of the physical mixtures of ibuprofen in Eudragit RS PO at different drug loadings in comparison to their equivalent hot melt extrudates. The diffraction peaks appeared in the physical mixtures over the concentration range 1-35% (w/w) of ibuprofen were absent in all diffract grams of the equivalent extrudates, which showed broad halos, indicating amorphicity of the extruded systems. However, at the higher ibuprofen content of 40% (w/w), extruded system showed clear diffraction peaks related to the crystalline ibuprofen (Figure 6b).
Microscopic examination using SEM of the fresh hot melt extruded systems of ibuprofen and Eudragit RS PO is shown in Figure 7. The surfaces and cross sections of these extruded systems up to 35% (w/w) drug loading were observed with no visible drug crystals, supporting drug miscibility within the Eudragit RS PO matrices. However, ibuprofen crystals were visible on both the surface and the cross section of the extruded system at 40% (w/w) drug loading, suggesting that ibuprofen content exceeded the drug solubility in the polymer Interestingly, increasing drug loading enhanced the smoothness of the surfaces and the elasticity during the cutting of the extruded systems, which again may be ascribed to ibuprofen plasticizing effect.
ATR-FTIR spectroscopy was used to assess possible interactions in the hot-melt extruded systems, which could have impact on the solubility, particularly at high drug loadings. Therefore, spectra of Eudragit RS PO, crystalline ibuprofen, amorphous ibuprofen, extruded mixtures of them at different drug loadings are shown in Figure 8.

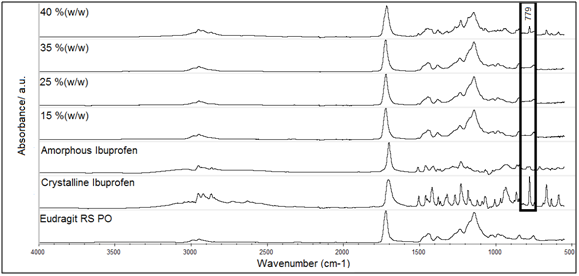

The reference spectra of the ibuprofen in Figure 8 showed that peaks in the region of 900-550 cm-1, due to the bending vibrations of aromatic ring, can be used to mark the differences between the amorphous and crystalline state of this drug. In particular absorbance band around 779 cm-1, due to the (=C-O-bending) of carboxylic acid.60 The ATR-FTIR spectra of the extruded systems of ibuprofen in Eudragit RS PO are also presented in Figure 8. It is noteworthy that spectra of the surfaces of the extruded systems are effectively identical to their cross sections (data not shown here) at all drug loadings, indicating possible homogeneity of the extruded formulations.61 The carbonyl stretch modes appear around 1722-1717cm-1 in all the equivalent physical mixtures (data not shown), associated with the stretching of the carboxyl group of the ibuprofen (1708 cm-1) and the ester group (C-CO-C) of the Eudragit RS PO at 1145 cm-1. These modes did not change in the corresponding extrudates, indicating little or no molecular interaction between the two species. Notably, the Eudragit RS PO bands hid most of the peaks of ibuprofen in the extruded systems in comparison to their physical mixes in the 1450-550 cm-1. region. However, the breadth (bandwidth) of these bands was slightly greater relative to the Eudragit RS PO, suggesting the presence of diffused (broadened) amorphous ibuprofen bands. This hypothesis is supported by the appearance of characteristic crystalline peak of ibuprofen at 40% (w/w) drug loading in these extrudates as highlighted in Figure 8. Thus, when the concentration of the drug exceeded the miscibility in the polymer, the ibuprofen recrystallized and its bands become more distinguished (sharpened) and appeared in this region (1450-550 cm-1) similar to the physical mix, which otherwise contains crystalline ibuprofen. Overall, these observations did not indicate a strong level of molecular interactions between the drug and the polymer after extrusion. However, they confirmed that all freshly prepared extruded samples contain the amorphous ibuprofen within Eudragit RS PO up to 35% (w/w) as shown previously by PXRD and DSC.
Influence of Relative Humidity on the Miscibility of Ibuprofen in Eudragit RS PO Extrudates
Investigations into the phase separation of hot melt extruded ibuprofen in Eudragit RS PO after storage at 0% RH: On storage at 0% RH for one month, the aged samples of ibuprofen-Eudragit RS PO extrudates up to 35% (w/w) drug loading remained transparent. Figure 9 compares the measured glass transition temperatures of the aged extrudates with those observed just after extrusion. This indicates a slight increase of the glass transition temperature after ageing under dry conditions over the concentration range of 1-35% (w/w) ibuprofen loading, possibly reflecting residual water removal.
The PXRD results supported the MTDSC data; Figure 10 shows an overlay of PXRD diffractograms of the aged extruded samples at different ibuprofen loadings. Compared to the fresh samples studied in the previous section (Figure 6b), the PXRD patterns over the concentration range of 1-35% (w/w) drug loading gave a similar characteristic amorphous halo, thus no indication of crystallinity. However, extruded samples at 40% (w/w) ibuprofen loading showed a visible increase in the resolution of the characteristic ibuprofen peaks, indicating an increase in preponderance of the crystalline form.
Furthermore, the aged samples were examined under SEM. There were no visible crystalline ibuprofen in these samples similar to the fresh samples up to 30% (w/w) drug loading, although at 35% (w/w) drug loading, micro-crystallites were detected on the surface of these aged extrudates (data not shown). ATR-FTIR was also used to investigate this system. The absorption band at 779 cm-1, attributed to the crystalline form of ibuprofen, sharpened noticeably at the surface of these samples (data not shown). Therefore, ATR-FTIR confirmed the SEM observation, implying that ibuprofen had been recrystallized in the aged extruded system at 35% (w/w) drug loading.
Overall, the findings in this section indicate that the systems under study are stable under dry conditions for one month, with some evidence of surface recrystallization being apparent at 35% (w/w) loading. This is fairly surprising given the low values of Tg observed (Figure 8), whereby higher drug content samples would have been stored at or above their Tg values. While interaction might not be an absolute requirement to avoid crystallization,62 it may be suggested that high viscosity of Eudragit RS63 is responsible for restricting and preventing crystal growth.
Investigations into the saturation solubility of hot melt extruded ibuprofen in Eudragit RS PO after storage study at 60% relative humidity
Representative reversing heat flow signals of MTDSC data of these samples stored under elevated humidity conditions are given in Figure 11a-c. The glass transition temperature was assigned accordingly from these signals and notably it was lowered to values ranging from 31.7±0.1 °C and 9.6±0.2 °C for the fresh 10-30% ibuprofen loaded systems to 25.1±0.4 °C and 3.2±0.2 °C, respectively, before any recrystallization was detected. Water contents of approximately 1.14 wt. % and 0.84 wt. % for the 10% and 30% loaded systems, respectively, were measured using TGA after one month of storage at 60% RH/25 °C. This would imply the critical role of water as a plasticizer for these extruded samples, as even small amounts of water were sufficient to depress the glass transition temperatures of these systems. Furthermore, it was observed that a melting endotherm, ascribed to ibuprofen melting and hence prior recrystallization, appeared at drug loadings higher than 10% (w/w).
PXRD was also used to monitor ageing of these extruded systems. With the exception of extrudates with 40% (w/w) drug loading, there was no indication of crystallinity. This could reflect low levels of crystallinity in these aged extrudates that are below the detection limits of this technique. However, in agreement with the MTDSC experiments, ibuprofen crystals were visible under the SEM of the aged extruded systems with drug loadings higher than 10% (w/w) as illustrated in Figure 12. Overall, this section has indicated that the stability of the system to recrystallization is significantly compromised on storage at elevated humidity’s, despite the water uptake being low.
Fragility assessment of ibuprofen-Eudragit RS PO extruded systems
It was noted in the previous section that the dry extruded systems showed good stability up to 30% (w/w) drug loading, despite the storage temperature being around or higher than their glass transition temperature. Given that some glassy systems may show good kinetic stability around Tg, it was useful to examine fragility parameters of the binary extruded systems, as this parameter can indicate the molecular mobility of the system, in turn relating to the amorphous phase stability.
Extrudates loaded with 10-30% (w/w) ibuprofen were studied using conventional DSC at a range of scanning rates.64 The fictive temperature 65 is the extrapolated intersection of the pre-transition and post-transition DSC heat flow baselines66 and depends only on the previous cooling rate through the glass transition temperature (thermal history), regardless the heating rate used for its measurement.67 Therefore, Equation 6 can be used to correlate the fragility parameter with the fictive temperature as follows:64
...Eq. (6)
Where βc is the prior cooling rate, Tf is the fictive temperature measured in the heating cycle, Tf,ref is the reference fictive temperature and mΔh is the fragility parameter. The value of Tf,ref may be obtained from the fictive temperature of the cooling rate equivalent to reheating rate used (the limiting fictive temperature).67
By cooling at various rates: 2, 5, 10 and 20°C/min, followed by heating at a constant rate of 10 °C /min through the glass transition region, the variation of the Tf with the cooling rate was used to define an enthalpic fragility parameter, mΔh.68 The fictive temperatures measured for these extrudates are summarized in Table 1 and Figure 13 (one exemplar shown), whereby the fragility parameters can be estimated from the slopes of the resulted lines, determined using a least squares best fit.66 The estimated fragility parameters fall below 100 (around 89.9, 70.2 and 32.0 for 10%, 20% and 30% (w/w) drug loaded extrudates, respectively), resembling behavior of strong, non-fragile systems.69 Therefore, it is less likely for the studied extruded systems to show physical relaxation of their glassy state and ultimately crystallization of the drug if stored at temperatures below their glass transition temperatures. Overall, therefore, this assessment supports the suggestion of the favorable stability of these (dry) systems.
Figure 11 MTDSC reversing heat flow signals of hot melt extruded ibuprofen-Eudragit RS PO systems at (a) 10% (w/w) (b) 20% (w/w) and (c) 30% (w/w) drug loading, examined on 0 day (fresh) and after 10, 20, 30 days of storage at 60% RH/ 25 °C. Single-column fitting.

Drug Loading (%w/w) |
10% (w/w) |
20% (w/w) |
30% (w/w) |
Cooling rate |
Tf±s.d./ °C |
Tf±s.d./ °C |
Tf ±s.d./ °C |
2 °C/min |
19.6±0.5 |
16.7±0.5 |
9.3±1.1 |
5 °C/min |
21.7±0.2 |
17.8±0.3 |
12.0±0.2 |
10 °C/min |
22.1±0.1 |
19.5±0.2 |
16.5±0.6 |
20 °C/min |
22.6±0.4 |
20.3±0.7 |
16.9±0.2 |
Table 1 Summary of the fictive temperatures (Tf) of the fresh hot melt extruded ibuprofen-Eudragit RS PO films at different drug loadings.
Hot melt extrusion technology was utilized successfully for the development of solid solutions of ibuprofen at high loading (up to 30-35% w/w) incorporated in Eudragit RS PO, with a view to applicability as a Transdermal system. Two theoretical approaches were utilised to predict miscibility, both of which indicated unusually high miscibility (a figure of 46.8% was obtained from melting point depression approaches). This high miscibility was born out by practical measurements using thermal, spectroscopic, diffraction and imaging approaches, whereby systems containing 35% w/w drug showed no evidence of crystallisation following extrusion. In addition, a marked decrease in torque was noted on extrusion, reflecting the ibuprofen acting as a plasticizer and further indicting high compatibility between the two molecules despite their considerable differences in molecular weight. However, no evidence was detected for specific molecular interactions or complexation between the two species. On storage under dry conditions for one month, favourable stability was noted with some evidence for surface crystallisation at circa 35% drug loading. Storage at 60% RH showed a marked decrease in Tg and compromise of stability, despite water uptake being low. Fragility calculations also supported the observation of stability on storage. Overall, the study has shown that theoretical calculations, which potentially save considerable time and effort in terms of polymer selection for HME, do appear to support practical observations in the present case in terms of both miscibility and stability. There are several notable observations from the study, which are unusual within the field, including the high level of miscibility for this system, the favourable stability despite the low Tg when stored under dry conditions but the profound influence of relatively low levels of water uptake on both Tg and associated stability of the extruded systems. In a subsequent communication, we will outline how these characteristics may be exploited to develop an effective approach (based on thermodynamic activity) to Transdermal delivery of this drug using hot melt extrusion with subsequent concentration and flux enhancement.
The author would like to thank German Jordanian University for their financial support.
The author declares no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.