MOJ
eISSN: 2471-139X


Objectives: Melissa officinalis has antioxidant and antidepressant effects. Besides, it could be good in learning and memory due to its terpenoides. The possible role of Melissa officinalis on ethanol state-dependent learning was studied in adult nicotine-treated male mice.
Methods: As a model of memory, a single–trial step-down passive avoidance task was used. In this project, ethanol (0.25, 1g/kg) and nicotine (0.01, 0.1mg/kg) was administrated 30minutes before training and testing. Melissa officinalis extract (25mg/kg) was administrated 30minutes before testing, and then step down latency (SDL) was measured.
Key finding: The obtained results showed that administration of ethanol (0.25, 1g/kg) and nicotine (0.1mg/ kg) before training could decrease SDL, whereas, nicotine (0.01mg/kg) increased SDL.
Conclusion: Pre testing administration of ethanol (1g/kg), nicotine (0.1mg/kg) and Melissa officinalis extract (25mg/kg) could ameliorate decreasing effects of pre training ethanol (0.25, 1g/kg) and nicotine (0.1mg/kg) on SDL.
Keywords: mellisa officinalis extract (varangboo), ethanol state dependent learning, nicotine-treated mice
SDL, step down latency; nAChRs, neuronal nicotinic acetylcholine receptors; DG, dentate gyrus; PSA-NCAM, polysialylated-neural cell adhesion Molecule; IP, intra peritoneally; AD, alzheimer’s disease; GABA, γ-amino butyric acid
State-dependent learning (SDL) is a well-established phenomenon now.1 The term is used to describe the finding that behavior learning in one drug state is better remembered when retention is tested in the same drug state.2 It is a well-known fact that ethanol, in view of its depressant effects,3 causes learning and memory deficits; it can however also exert facilitatory effects on memory.4 Alcohol and tobacco are the most commonly used addictive drugs in the world.5 Although there are many possible reasons for the co-abuse of nicotine and alcohol, one possible mechanism is that both nicotine and alcohol act at the level of the neurotransmitter receptor system, the neuronal nicotinic acetylcholine receptors (nAChRs).6 Alcohol dependence is usually accompanied by tolerance to the intoxicating effects of alcohol. Ethanol causes various dose-dependent behavioral effects in rodents, ranging from the stimulation of locomotors activity after low doses to motor impairment, hypothermia, sedation and loss of the righting reflex.7 In the mammalian brain, the hippocampus and the dorsal striatum support fundamentally distinct forms of memory.8 Tolerance to ethanol may be an important predictor of susceptibility to alcoholism because it may be a significant factor in the relationship between alcohol consumption and dependence.9 Nicotine is the neuroactive compound that is considered to be responsible for the development and maintenance of tobacco addiction.10 It was found that nicotine self-administration profoundly decreased the expression of PSA-NCAM (Polysialylated-neural cell adhesion molecule) and neurogenesis in the DG (dentate gyrus).11 Modifications of PSA-NCAM expression in mutant mice results in morphological modifications, impairment of cognitive function12 and perturbations of synaptic plasticity.13 In studies with human subjects, alcohol,14 marijuana,15 barbiturate, amphetamine,16 methylphenidate17 and nicotine have all been shown to produce SDL effects.18 Tolerance to ethanol may be an important predictor of susceptibility to alcoholism because it may be a significant factor in the relationship between alcohol consumption and dependence.19 Lemon balm, Melissa officinalis L. (Lamiaceae), is an herb of long tradition and with a large variety of uses.20 Melissa officinalis leaves contain polyphenolic compounds, such as rosmaric acid, trimeric compounds and some flavonoids.21 It can scavenge free radicals and have antioxidant properties.22 Melissa officinalisL. (Labiatae) has been frequently used in Iranian traditional medicine to treat neurological disorders such as depression and anxiety, and it is also mentioned as a memory enhancing herb. Melissa officinalis also enhances memory and relieves stress.23 The aim of followed project is to study effects of Melissa officinalis on ethanol state-dependent learning in nicotine- treated mice.
Animals
Mice weighing 25-30g were purchased from the Pasteur Institute of Iran, housed in groups of six in stainless-steel cages, and given food and water ad libitum under a standard 12h light/12h dark cycle. All training and test sessions were performed in a glass room where only the wooden platform was placed in (standard conditions) middle of the box. Four groups of animals received saline 30minutes before training and saline, nicotine (0.01, 0.1mg/kg), ethanol (1, 0.25g/kg) and Melissa officinalis extract (25mg/kg) before testing. Other groups received ethanol( 0.25g/kg) 30minutes before training and ethanol (0.25g/kg) before testing, two groups, 30minutes before training received ethanol (0.25, 1g/kg) and nicotine (0.1, 0.01mg/kg). These groups also received saline before testing. Three groups received ethanol (0.25g/kg), nicotine (0.1mg/kg) and both of them 30minutes before training and Melissa officinalis extract (25mg/kg) before testing.
Behavioral procedures
Training: In the training day, each mice received nicotine or ethanol intra peritoneally (IP) and then 30min after injection each mice was gently placed on the platform. Five seconds 0.4mA shock was applied to the grid after which animals were immediately withdrawn from the training apparatus. This training procedure was carried out.
Retention test: Twenty-four hours after training, step-down latency was measured 30min after the last injection. Each mice was gently placed on the platform, without any shock. The step-down latency (SDL) was taken as a measure of retention.
Task: The wood escape platform used for the spatial task at the middle of the glassy box. An electric shock (0.4 mA, 5s) was delivered to the grid floor by an isolated stimulator.
Plant material and extraction procedure
The total plant extract was obtained by extraction of dried and milled plant leaves with ethanol 70% (1:10) using the maceration method for 4days. After every 24h, the mixture was filtered, and fresh solvent was added to the plant powder. The combined extracts were concentrated to dryness.
Pre- training and pre- testing effect of ethanol on step down latency (SDL)
Figure 1 shows the effect of pre-training injection of ethanol. In animals that received ethanol (0.25 , 1g/kg) before training, significant decrease was observed compared with control group (saline) (**P<0.01) on the step-down latencies (SDL) In pre-training injection of saline and pre-testing injection of ethanol (0.25g/kg), significant increase was observed compared with control group (saline) (*P<0.0 5) on the step-down latencies (SDL). Two groups of animals received pre- training injection of higher dose of ethanol (1g/kg) and pre-test injection of different doses of ethanol (0.25, 1g/kg). In that animals in which SDL was impaired due to pre-training administration of ethanol (0.25, 1g/kg), pre-test administration of ethanol (1g/kg) restored SDL to control level .
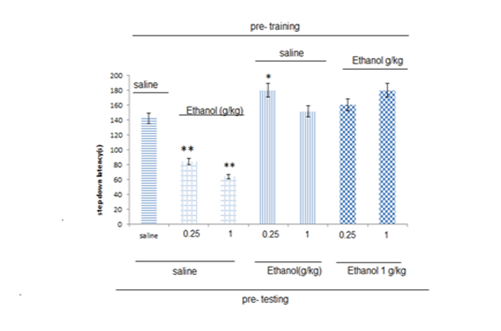
Figure 1 The effects of ethanol on inhibitory avoidance memory.
Seven groups of animals were used. Five groups of animals received Saline, or different doses of ethanol (1, 0.25g/kg) before training and testing. Two groups of animals received pre-training and pre testing injections of ethanol (1, 0.25g/kg). Data are the mean±SE.
*Significant difference with saline + saline group.
Pre-training and pre-testing effect of nicotine on step down latency (SDL)
Figure 2 shows in animals that received nicotine (0.01 and 0.1mg/kg) before training compared with control group (saline), significant increase and decrease was observed respectively (*P<0.05), (***P<0.001) in the step-down latencies (STD). Effect of pre-testing administration of nicotine in animals that received nicotine (0.1mg/kg) compared with control group (saline) significant decrease was observed (**P<0.01). Furthermore, in the animals that received ethanol (1, 0.25g/kg) before training and nicotine (0.01mg/kg) on the test day, compared with control group (saline), significant increase was observed in the step down latencies (SDL) in lower dose of ethanol(0.25g/kg) (*P<0.05).
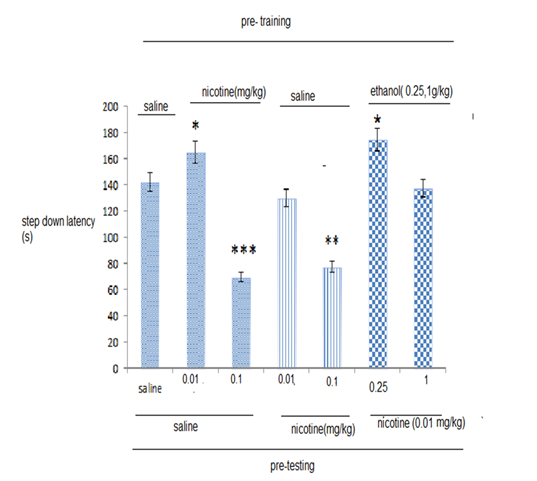
Figure 2 The effects of nicotine on inhibitory avoidance memory.
Seven groups of animals were used. Five groups of animals received pre-training and pre testing, saline, or different doses of nicotine (0.1, 0.01mg/kg). Two groups of animals received pre-training injections of ethanol(1, 0.25g/kg) and nicotine (0.01mg/kg) on the test day. Data are the means±SEM.
*Significant difference with saline + saline group.
Pre-training ethanol, nicotine and pre-testing varangboo effects on step down latency (SDL)
As Figure 3 shows animals that received ethanol (0.25g/kg) before training and Varangboo before testing compared with a group which received ethanol before training and saline before testing˲ a significant increase was observed (##p<0.01). A group that received nicotine (0.1mg/kg) before training and Varangboo before testing compared with a group which received nicotine (0.1mg/kg) before training and saline before testing, a significant increase was observed (p<0.05). Animals received nicotine (0. 1mg/kg) plus ethanol (0.25g/kg) before training and Varangboo (25mg/kg) before testing compared with ethanol+Varangboo group˲ a significant increase was observed (n¾p<0.01) and in compared with nicotine+varangboo significant difference was not observed. In other comparison between ethanol+nicotine+varangboo with saline+varangboo. Significant increase was observed (ΔP<0.05) on SDL.

Figure 3 Pre-training ethanol, nicotine and pre-testing Varangboo effects on step down latency (SDL).
Three groups of animals received saline, ethanol (0.25g/kg) and nicotine (0.1mg/kg) before training and saline before testing, four groups of animals received saline, ethanol (0.25g/kg), nicotine (0.1mg/kg) and ethanol (0.25g/kg) + nicotine(0.1mg/kg) before training and varangboo (25mg/kg) before testing. Data are the means±SEM.
*Significant difference with saline+saline group
#Significant difference with ethanol+saline group
●Significant difference with nicotine+saline group
vSignificant difference with ethanol+Varangboo group
Δ Significant difference with saline+varangboo group.
Cholinergic neurons in the basal forebrain and hippocampus, and acetylcholine as a major neurotransmitter in these neurons, have important roles in learning and memory processes.24 Our present data showed that pre-training administration of ethanol (1, 0.25g/kg) decreased inhibitory avoidance memory. Alcohol can have a severe disruptive influence on human memory.25 According to the previous studies mental dysfunction observed after chronic ethanol consumption, can largely be attributed to a degeneration of the cholinergic pathway of the ascending activation system resulting in an impairment of cortical activation, clinically appearing as the “syndrome of partial cholinergic differentiation of the cortical mantle.26 The response to acute ethanol may be due to increase in GABA-mediated inhibition or decrease in NMDA-evoked hippocampal neuronal activity.27 In this project, when ethanol used on the test day, increased inhibitory avoidance memory was observed. This phenomenon was named ethanol state-dependent learning which was previously studied.28 The term is used to describe the finding that behavior learned in one drug state is better remembered when retention is tested in the same drug state.2 The present results also show that, the pre-train and pre- test administration of ethanol also reversed the decrease in inhibitory avoidance response induced by ethanol. Studies suggested that the inhibition of hippocampal ACh release by intoxicating doses of ethanol may contribute to the well-known cognitive and amnesic effects of ethanol intake.29 Acute ethanol administration produces dose-dependent impairments in spatial learning. Ethanol also decreases the spatial specificity of hippocampal place cells. Such findings raise the possibility that ethanol affects learning and memory by altering, either directly or indirectly, neuronal activity in the hippocampus and related structures.30 In this study also indicated that pre-training administration of nicotine (0.01mg/kg) increased inhibitory avoidance memory in spite of higher dose of nicotine (0.1mg/kg). When a particular dose of nicotine is exceed, nicotine-induced responses diminish rapidly. It is specific to the higher nicotine doses, which induce little neuronal activation in many neuronal structures, e.g., the NAC, amygdale, and other limbic areas such as the septum, hippocampus, and hypothalamus. Responsible for this nicotine biphasic pattern of action is acute nicotine tolerance and related desensitization of nicotinic receptors.31 Involvement of neuronal nicotinic cholinergic systems in learning and memory processes has been recognized for several decades.32 Evidence suggests that nicotinic acetylcholine receptor (nAChR) agonists ameliorate the cognitive decline associated with schizophrenia and Alzheimer’s disease (AD) progression.33 Nicotine ability to enhance memory and learning it seems to result from activation of central nAChRs in the prefrontal cortex, hippocampus, and amygdale.34 Activation of these receptors provokes the release of several neurotransmitters, including dopamine, nor adrenaline, 5-HT, ACh, gamma-amino butyric acid, glutamate, and histamine,35 important in the regulation of memory processes. Investigation showed that nicotine appear to elevate [Ca2+]i by promoting the influx of extracellular Ca2+ through voltage-gated calcium channels.36 Activation of atrocities receptors surrounding a single synapse causes local increases in calcium.37
In addition, atrocities have been shown to participate in calcium-mediated vesicular release of glutamate that can modulate neuronal activity.17,18 Nicotine activates nAChRs in the mesocorticolimbic dopaminergic system that projects from the ventral tegmental area to the nucleus accumbens and the prefrontal cortex.38 There are also reports indicating that nicotinic and NO systems have interactions.39 In some other studies, the inhibitory effects of NOS inhibitors on the behavioral effects of nicotine have been shown. For example, it has been indicated that NOS inhibitors block the development of behavioral sensitization to nicotine,40 nicotine-induced conditioned place preference41 and suppress signs of withdrawal from nicotine.42 The present data also show that pre-training administration of ethanol ameliorate cognitive decline associated with pre-testing administration of nicotine. Nicotine, unlike ethanol enhances learning through a direct effect on attention or through interacting with pre-synaptic nicotinic acetylcholine receptors (nAChR). Nicotine facilitate the release of many neurotransmitters such as acetylcholine, glutamate, dopamine, nor epinephrine, serotonin and γ-amino butyric acid (GABA), all of which are critical to normal learning and memory function.43 Since ethanol and nicotine have some opposite effects on cognitive functions,44 the interaction between them is complex and not fully understood yet. Considering that ethanol and nicotine have functional interactions with glutamate45 and the dorsal hippocampus is a key structure in learning and memory.46 The ethanol-nicotine interaction on learning and memory have also been demonstrated by some neurobehavioral investigations.47 Advances are also being made in identifying and understanding the neurobiological mechanisms that mediate genetic risk for comorbid alcohol and tobacco dependence. For example, Owens et al. (2003) found strong evidence that sensitivity to the effects of both nicotine and alcohol on acoustic startle in mice is mediated by polymorphisms in genes that code for nicotinic acetylcholine receptors (nAChRs). An association between polymorphisms in these genes and sensitivity to both alcohol and cigarettes has also been found in a human study.48 Polymorphisms in other receptor systems, including the dopaminergic, gamma-amino butyric acid, and opioid systems, may also account for individual differences in sensitivity to alcohol and nicotine.49 The main finding of this experiment is that Mellisa officinal is extract can increase memory. The obtained results showed that injection of pre-training of nicotine (0.1mg/kg) and ethanol (0.25g/kg) and pre-testing of Mellisa officinalis extract 25mg/kg Intra peritoneally can increase memory. Herbal extracts include several materials with heterogeneous pharmacological effects were attended for complex situation like AD.50 The studies suggested that ethanol-induced memory impairment can be ameliorated by pharmacological manipulation of central cholinergic function. The extract of Mellisa officinalis has a cholinergic property.51 In the present study, it was found that when Varangboo (25mg/kg) injected before testing in groups that received ethanol (0.25g/kg) or nicotine (0.1mg/kg) before training, it could reversed their decreasing effect on SDL. Current research has shown that this herb can calm the patients in their behavior, improve their learning, and enhance their short-term memory.52 M. officinalis extract showed some nicotinic and muscarinic activity.53 Also, it has been reported that M. officinalis extract has nicotinic receptor activity and that it can displace [3H]-(N)-nicotine from nicotinic receptors in homogenates of human cerebral cortical cell membranes.51 Old European reference books (eg. medical herbals) document a variety of other plants such as Salvia officinalis (sage) and Melissa officinalis (balm) with memory improving properties, and cholinergic activities have recently been identified in extracts of these plants.54 As Table 1 shows rosmaric acid is the more part of component. The results indicate the beneficial effects of sub chronic RA administration in passive avoidance learning and memory.55 The anti-cholinesterase activity of M. officinalis extract and its main constituent rosmarinic acid was reported previously.56
The present study showed that M. officinalis extract can improve in ethanol and nicotine-treated mice.
None.
Author declares that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.