
Correspondence:
Received: January 01, 1970 | Published: ,
Citation: DOI:
Download PDF
Over 30 million individuals are infected with HIV-1 world-wide. The disease produces great human suffering and extracts an enormous financial toll. Although antiretroviral drugs have produced substantial reductions in HIV-1 related morbidity and mortality, they are costly, associated with side effects, and ineffective in the presence of drug resistant virus. Despite intensive efforts, both a protective vaccine and a cure for HIV-1 remain elusive.
Ongoing HIV-1 replication despite host immune responses to the virus is an enigma and poses a major barrier to the development of a vaccine or a cure. HIV-1 replication occurs primarily in CD4+T cells within secondary lymphoid tissues [1,2] and results in a progressive decline of CD4+T cells, immunodeficiency, and ultimately death in most untreated individuals. Within secondary lymphoid tissues, HIV-1 replication is highly concentrated in follicular CD4+T cells [3-10], which are 30-40 times more likely to be productively infected than extra follicular CD4+T cells during chronic HIV-1 infection [3]. Consequently, follicular CD4+T cells account for approximately 70% of HIV-1 producing cells in chronic disease prior to AIDS [3,4]. Follicular dendritic cells (FDC) located in germinal centers of follicles, bind HIV-antibody complexes, and these complexes readily infect CD4+T cells in vitro [11], thus providing an explanation for the high rate of infection of follicular CD4+T cells. Nevertheless, it is unclear why HIV-1-specific CD8+T cells are unable to fully suppress HIV-1 replication in follicular CD4+T cells, as they are able to kill productively infected cells in vitro within minutes after they initiate transcription of virus and long before infectious virions are produced.
Multiple lines of evidence indicate that HIV-1-specific CD8+T cells play a pivotal role in controlling virus replication. Development of HIV-1-specific CD8+T cells during acute infection coincides with declines in viremia [12-14] suggesting that CTL are critical determinants of the initial control of virus replication. In the SIV-infected rhesus macaque model of HIV-1, temporary removal of CD8+T cells leads to increased viremia, and the subsequent return of CD8+T cells correlates with decreased viremia [15,16], further implicating CD8+T cells in viral control. Levels of polyfunctional CD8+T cells inversely correlate with virus set point [17] and polyfunctional CD8+T cells are maintained in HIV-1 infected nonprogressors [18]. Furthermore, there is a strong association of MHC class-I alleles with particular outcomes of HIV-1 and SIV infections [19], and it is hypothesized that this is related to the efficiency of virus-specific CTL responses. The CD8+T cell response to HIV-1 is unique because in some cases virus-specific cytolysis is detectable in PBMC in the absence of in vitro stimulation [20,21], a phenomenon that has not been frequently reported in other chronic viral infections. Nevertheless, despite evidence that HIV-1-specific CD8+T cells are abundant and capable of cytolytic function, they are unable to fully suppress virus replication in vivo resulting in the progressive depletion of CD4+T cells and, ultimately, death in untreated individuals. Quite perplexingly as well, in multiple studies the administration of exogenous CD8+T cells has failed to result in significant decreases in plasma HIV-1 RNA concentrations [22-25]. Furthermore, increases in HIV-specific CTL through therapeutic vaccination or treatment interruption have resulted in little or no enhancement of virologic control [26-29].
HIV-1 evades HIV-1-specific CD8+T cells through multiple mechanisms, which likely contribute to the inability of HIV-1-specific CTL to fully control HIV-1 replication. First, HIV-1 latently infected cells do not express viral proteins, which are essential to CD8+T cell recognition. Second, HIV-1 frequently mutates to evade CD8+T cell responses [19]. Third, HIV-specific CD8+T cells fail to accumulate in lymphoid B cell follicles and are not able to effectively clear the follicular reservoir of HIV-1-producing cells [3] (Figure 1). This might explain why individuals with functional HIV-specific CD8+T cells fail to fully suppress HIV-1 replication, and why infused exogenous CD8+T cells or augmented endogenous CTL responses failed to significantly impact viral control.
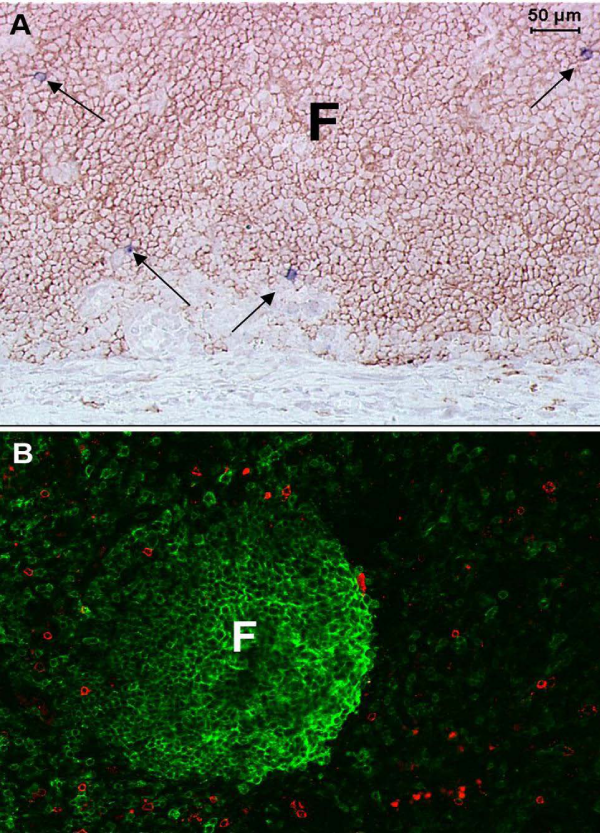
Figure 1: In lymph nodes from untreated HIV-1-infected individuals, virus replication is largely concentrated inside B cell follicles, whereas HIV-1-specific CD8+T cells fail to accumulate at those sites. A) HIV-1 RNA+ cells identified by in situ hybridization (blue-black staining cells indicated by arrows) are located primarily inside of a B cell follicle, defined morphologically by staining with anti-CD20 antibodies (brown). B) HIV-1-specific CD8+T cells labeled in situ with HLA-tetramers (red) are concentrated primarily outside of a B cell follicle, defined by anti-CD20 antibodies (green).
Future HIV-1 cure and vaccine strategies must address not only the latent reservoir of HIV-1 infected cells and HIV-specific CD8+T cell escape mutations, but also the reservoir of HIV-1 producing cells within B cell follicles. Strategies to induce follicular lysis, such as through administration of the B-cell depleting monoclonal antibody rituximab, might temporarily allow virus-specific CTL to access the follicles. However, this would profoundly impair humoral immunity, and furthermore, the effects would only be transient. Once the B cell population recovered and follicles were reconstituted, HIV-1 replication most likely would resume at that site. Perhaps a better approach to eradicate the reservoir of HIV-1 within follicles, and one that we are presently developing [30], is to engineer functional HIV-1-specific CD8+T cells to express chemokine receptors, such as the follicular homing molecule CXCR5, to allow virus-specific CTL to access the follicular compartment and clear the reservoir of HIV-1 producing cells within follicles. This approach could be applied by itself or in conjunction with other approaches such as one to stimulate latently infected cells to express HIV-1. Additionally, this approach could be implemented using CD8+T cells directed against HIV-1 neo-epitopes [19], in cases where HIV-specific CD8+T cell escape mutations are an issue. Ultimately, therapeutic approaches that enable HIV-specific CD8+T cells to enter lymphoid B cell follicles and clear HIV-1 producing cells may lead to a functional cure for HIV-1.
Acknowledgment
This work was supported by Public Health Services Grant R01AI096966 from the National Institutes of Health.
References
- Embretson J, Zupancic M, Ribas JL, Burke A, Racz P, et al. (1993) Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature 362(6418): 359-362.
- Pantaleo G, Graziosi C, Demarest JF, Butini L, Montroni M, et al. (1993) HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 362(6418): 355-358.
- Connick E, Mattila T, Folkvord JM, Schlichtemeier R, Meditz AL, et al. (2007) CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol 178(11): 6975-6983.
- Folkvord JM, Armon C, Connick E (2005) Lymphoid follicles are sites of heightened human immunodeficiency virus type 1 (HIV-1) replication and reduced antiretroviral effector mechanisms. AIDS Res Hum Retroviruses 21(5): 363-370.
- Biberfeld P, Chayt KJ, Marselle LM, Biberfeld G, Gallo RC, et al. (1986) HTLV-III expression in infected lymph nodes and relevance to pathogenesis of lymphadenopathy. Am J Pathol 125(3): 436-442.
- Hufert FT, van Lunzen J, Janossy G, Bertram S, Schmitz J, et al. (1997) Germinal centre CD4+ T cells are an important site of HIV replication in vivo. AIDS 11(7): 849-857.
- Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, et al. (2004) Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med 200(6): 761-770.
- Orenstein JM (2008) HIV expression in surgical specimens. AIDS Res Hum Retroviruses 24(7): 947-955.
- Tenner-Racz K, Racz P (1995) Follicular dendritic cells initiate and maintain infection of the germinal centers by human immunodeficiency virus. Curr Top Microbiol Immunol 201: 141-159.
- Tenner-Racz K, Stellbrink HJ, van Lunzen J, Schneider C, Jacobs JP, et al. (1998) The unenlarged lymph nodes of HIV-1-infected, asymptomatic patients with high CD4 T cell counts are sites for virus replication and CD4 T cell proliferation. The impact of highly active antiretroviral therapy. J Exp Med 187(6): 949-959.
- Heath SL, Tew JG, Tew JG, Szakal AK, Burton GF (1995) Follicular dendritic cells and human immunodeficiency virus infectivity. Nature 377(6551): 740-744.
- Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB (1994) Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol 68(9): 6103-6110.
- Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, et al. (1994) Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol 68(7): 4650-4655.
- Connick E, Marr DG, Zhang XQ, Clark SJ, Saag MS, et al. (1996) HIV-specific cellular and humoral immune responses in primary HIV infection. AIDS Res Hum Retroviruses 12(12): 1129-1140.
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, et al. (1999) Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283(5403): 857-860.
- Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, et al. (1999) Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med 189(6): 991-998.
- Riou C, Burgers WA, Mlisana K, Koup RA, Roederer M, et al. (2014) Differential impact of magnitude, polyfunctional capacity, and specificity of HIV-specific CD8+ T cell responses on HIV set point. J Virol 88(3): 1819-1824.
- Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, et al. (2006) HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107(12): 4781-4789.
- Goulder PJ, Watkins DI (2008) Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat Rev Immunol 8(8): 619-630.
- Walker BD, Chakrabarti S, Moss B, Paradis TJ, Flynn T, et al. (1987) HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature 328(6128): 345-348.
- Ferbas J, Kaplan AH, Hausner MA, Hultin LE, Matud JL, et al. (1995) Virus burden in long-term survivors of human immunodeficiency virus (HIV) infection is a determinant of anti-HIV CD8+ lymphocyte activity. J Infect Dis 172(2): 329-339.
- Koenig S, Conley AJ, Brewah YA, Jones GM, Leath S, et al. (1995) Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat Med 1(4): 330-336.
- Lieberman J, Skolnik PR, Parkerson GR, Fabry JA, Landry B, et al. (1997) Safety of autologous, ex vivo-expanded human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte infusion in HIV-infected patients. Blood 90(6): 2196-2206.
- Mitsuyasu RT, Anton PA, Deeks SG, Scadden DT, Connick E, et al. (2000) Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood 96(3): 785-793.
- Brodie SJ, Lewinsohn DA, Patterson BK, Jiyamapa D, Krieger J, et al. (1999) In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat Med 5(1): 34-41.
- Autran B, Murphy RL, Costagliola D, Tubiana R, Clotet B, et al. (2008) Greater viral rebound and reduced time to resume antiretroviral therapy after therapeutic immunization with the ALVAC-HIV vaccine (vCP1452). AIDS 22(11): 1313-1322.
- Kinloch-de Loes S, Hoen B, Smith DE, Autran B, Lampe FC, et al. (2005) Impact of therapeutic immunization on HIV-1 viremia after discontinuation of antiretroviral therapy initiated during acute infection. J Infect Dis 192(4): 607-617.
- Goujard C, Marcellin F, Hendel-Chavez H, Burgard M, Meiffredy V, et al. (2007) Interruption of antiretroviral therapy initiated during primary HIV-1 infection: Impact of a therapeutic vaccination strategy combined with interleukin (IL)-2 compared with IL-2 alone in the ANRS 095 randomized study. AIDS Res Hum Retroviruses 23(9): 1105-1113.
- Markowitz M, Jin X, Hurley A, Simon V, Ramratnam B, et al. (2002) Discontinuation of antiretroviral therapy commenced early during the course of human immunodeficiency virus type 1 infection, with or without adjunctive vaccination. J Infect Dis 186(5): 634-643.
- Haran P, Rakasz E, Kaushik K, et al. (2014) Transduction of primary rhesus macaque CD8 T cells with the B cell homing molecule CXCR5. Keystone Symposia: HIV Pathogenesis, Banff Canada.

© . This is an open access article distributed under the terms of the,
which
permits unrestricted use, distribution, and build upon your work non-commercially.




 World Malaria Day, observed annually on April 25SUP>th. The purpose of this day is to raise awareness about the global struggle to combat malaria, highlighting the need for continued research, prevention, and treatment strategies to eradicate this deadly disease. So, it is an opportunity to all the researchers to submit your interesting papers on raising the approachability on modern vaccine development, advances in disease control, prevention and eradication and the submissions received till April 25th, 2024 will be offered a best discount of 30% for publication in Journal of Human Virology & Retro virology.
World Malaria Day, observed annually on April 25SUP>th. The purpose of this day is to raise awareness about the global struggle to combat malaria, highlighting the need for continued research, prevention, and treatment strategies to eradicate this deadly disease. So, it is an opportunity to all the researchers to submit your interesting papers on raising the approachability on modern vaccine development, advances in disease control, prevention and eradication and the submissions received till April 25th, 2024 will be offered a best discount of 30% for publication in Journal of Human Virology & Retro virology.