Journal of
eISSN: 2377-4312


The present study was carried out to investigate the possible etiology and pathology of cystic ovarian follicles by determining the expression levels of mRNA for LH-r gene in follicular cells of dominant follicles in comparison with cystic ovarian follicles in Iraqi cows. This study were performed in two steps, firstly aspiration of follicular fluids from dominant follicles and cystic follicles and stored in (-20℃) until estradiol and progesterone assay, secondary section of the follicular wall in to two hemispheres, and stored at -70℃ to -80℃ to molecular study. The macroscopic examination of the ovaries revealed that the numbers of dominant follicles are1 samples while cystic follicles were in samples.2 There was a significant difference (P<0.05) in the diameter of cystic ovarian follicles (37.56±0.64mm) compared with dominant follicles (19.93±0.32mm).
Results of hormonal assay showed higher estradiol-17β (865.96±10.64ng/ml) and progesterone (84.8±1.35ng/ml) concentrations in follicular fluids of cystic ovarian follicles, which were significantly higher (P<0.05) in comparison with those of dominant follicles which were (314.39±2.55ng/ml) and (50.25±1.57ng/ml) respectively. Molecular study, to evaluate the relative quantification of LH-r gene in dominant and cystic follicular cells, has been done by extraction of the total RNA and assay its concentration from these cells, synthesis data of the complementary DNA (cDNA), that done by reverse transcription PCR (q-RT-PCR) technique, of target gene and compared of the gene expression in dominant and cystic follicular cells, the our results referred to down regulation of LH-r gene expression in follicular cells of the cystic ovarian follicles, that may be assistance to understand the etiology and pathology of this case (disease), thus the up-regulation of the this gene in cells of dominant follicles may explain the important role of the LH in ovulation mechanism and increasing follicular ovulation chance.
Keywords: gene expression, cows, ovarian follicles, LH-r gene
cDNA, complementary DNA; E2, estradiol-17β; P4, progesterone; q-RT-PCR, reverse transcription PCR; FF, follicular fluid; GADPH, glyceraldehyde-3-phosphate dehydrogenase; PBS, phosphate buffer saline; DF, dominant follicles; COF, cystic ovarian follicles; CL, corpus luteum; COF, cystic ovarian follicles; RIA, radio-immuno assay
Cystic ovarian follicle(COF) a serious cause of the reproductive failure in cattle because they occur frequently and prolong the intervals from postpartum to first estrus and conception.3 It’s a larger than a pre-ovulatory follicle (>25mm in diameter) and persists for ten or more days in the absence of a corpus luteum.4 The COF characterized by thin wall and accumulation of an excess amount of follicular fluid (FF) inside the follicle which contains of many components, including hormones like estradiol-17β (E2) and very small amounts of progesterone (P4). The FF also contain of proteins like glyceraldehyde-3-phosphate dehydrogenase (GAPDH) with its receptors.5
The follicle health and steroidogenic status of the follicular cyst were determined by measurement of the E2 and P4 concentration in follicular fluid (FF).6 Beg MA7 Indicate that the future DF acquired LH-r before follicular deviation, whereas others like Fortune JE et al.8 and Barros CM et al.9 reported that LH-r expression occurred after follicular deviation, yet confirmed that hypothesis of higher follicle diameters lead to increase the gene expression of LH-r in granulosa cells.
The up-regulation of expression of genes for LH-r in granulose cells and down-regulation of expression of genes for FSH-r were associated with Growth of follicles until become of graafian follicles.7 There are many studies like Ginzinger DG10 and Barber RD et al.11 used this technique to explained the gene expression levels and measured the mRNA transcript levels in a quantitative fashion by combining the two technologies real-time PCR with reveres transcription (real time-PCR). The relative quantification describes the change in expression of the target gene relative to some reference gene group such as (GAPDH) in eukaryotic cells to normalizing q(rt-PCR) data,12 yet there are three ways to application of the relative quantification methods like Livak method (2^ΔΔCt method), ΔCt method by using the reference gene, and Pfaffl method Livak KJ13 and Pfaffl MW.14
Quantitative Reverse Transcriptase Real-Time PCR Kits
All kits which used in quantification of gene expression levels by qRT-PCR, and hormonal kit which used to RIA method, with their companies and countries of origin (Table 1).
Primers
Two set of primers are used in this study, first primer used for GAPDH gene as Housekeeping gene and other primer used for LH-r gene as target gene. These primers were designed by using NCBI- Gene Bank data base and Primer 3 design online, the primers used in quantification of gene expression using quantitative (real time-PCR) techniques based SYBER Green DNA binding dye, and supported from (Bioneer, Korea) company (Table 2).
Collection of the follicles
The presence study depends on collection of ovaries from sexually mature cows (4-6years) old with unknown reproductive status slaughterhouses during December 2011 to October 2012, these ovaries transported within 1-2 hours in cool box (ice) to the vet. laboratory, then each ovaries was subjected to washing in phosphate buffer saline (PBS) and one washing in ethanol 70%, examined by macroscopic exam (color, consistency, corpus luteum (CL) stage, follicular numbers and follicular size) according to Berisha B.15 Its ovaries were divided in to two groups’ dominant follicles (DF) and cystic ovarian follicles (COF) were used in this study.
Dominant and Cystic Ovarian Follicles: The dominant follicles (DF) group with diameter range 17-25mm, contain only healthy follicles which having transparent fluids, and present of regress corpus luteum for previous estrus cycle.16 The cystic ovarian follicles (COF) are samples having large un-ovulated persistent follicles on the ovary and diagnosed by the macroscopic notation such as the follicle diameter are greater than 25mm, absent of any corpus luteum in both the right and left ovaries and the follicular walls of the cysts were thin and translucent.17
No. |
Kit |
Company |
Country |
1 |
AccuZol™ Total RNA ExtractionKit -Trizol 100ml |
Bioneer |
Korea |
2 |
AccuPower®RocktScript RT PreMix |
Bioneer |
Korea |
- RocketScript Reverse Transcriptase (200u) |
|||
-5×Reaction Buffer (1×)- RNase Inhibitor (1u) |
|||
-DTT (0.25mM)-Dntp (250µM each) |
|||
3 |
AccuPower® Greenstar™ qPCRPreMix |
Bioneer |
Korea |
- SYBER Green fluorescence - Exicycler™ 20µL reaction |
|||
-8 Well strips×12 each- DEPC – D.W. 1.8ml×4 tubes |
|||
4 |
EZ-10RNA Mini-Preps Kits Handbook -RNase-Free DNase |
Bio basic |
Canada |
Set |
|||
5 |
Immunotech RIA Progesterone (kit) |
Beckman Coulter |
France |
6 |
Immunotech RIA Estradiol (kit) |
Beckman Coulter |
France |
Table 1 Quantitative Reverse Transcriptase Real-Time PCR Kits
Study design
These two groups DF and COF have been done in two steps which are
Estradiol-17β and progesterone assay in follicular fluid
The FF was aspirated from DF and COF, and collected separately in test tubes contain anticoagulant, then centrifugation 2000rpm for 10minutes and stored at -20℃ until hormonal assay, as a described previously by Vanholder T.18
Isolation of the follicular cells
Isolation of the follicular cells from the follicular frozen wall part (dominant or cyst) which are comprise from granulosa and theca cells according to Nogueira MF,19 the follicular cell mashed was stored at -70℃ to -80℃ in deep freeze system until total RNA extraction according to flowing steps.
Total RNA extraction: The total RNA was extracted from a follicular cell pellet using of the total RNA extraction reagent (Accuzol® Usere manual, BIONEER-Korea) and according to the manufacturer’s instructions.
Quantification of total RNA: Quality control standards were applied to all RNA samples in this study; these were that the purity was 1.7-1.9, total RNA samples were adjusted at same concentrations. This is performing by nanodrop spectrophotometer machine (OPTIZEN POP. MECASYS KOREA).
DNase Treatment: Regarding to the disadvantage of the SYBR green I, which it binds to any double-stranded DNA and produce of non-specific primer-dimers. Then treatment the extracted total RNA by DNase enzyme to remove the trace amounts of genomic DNA by using (DNase I enzyme), according to company instructions (BIOBASIC, USA).
cDNA Synthesis: Reverse transcription-PCR control was performed with primers for GAPDH to check the removal of all the contaminating genomic DNA. First-strand cDNA was synthesized from 1500ng of RNA using the cDNA synthesis kit (AccuPower® RocktScript RT PreMix), following the manufacturer’s instructions.
Quantitative (real time-PCR)
According to method described by Wang G,20 calculated the relative expression by q (rt-PCR) for target gene LH-r gene in follicular cells of DF in comparison with COF, the ΔCT USING A REFERENCE GENE METHOD can be used by normalizing gene expression of target gene (LH-r) with gene expression of housekeeping gene (GAPDH) as a reference gene.
This method used the difference between reference and target Ct values for each sample, the expression level of the reference gene are taken into account using following formula:
Expression value (fold yield)=2^CT (reference)–CT (target)
Two-Step (real time-PCR): The two-step reaction, revere to the reverse transcription amplification occur in separate tubes (two-step rt-PCR which mean separates the reverse transcription reaction from the rt-PCR assay), two-steps protocol may be preferred when using a DNA binding dye (such as SYBR Green I) because it’s easier to eliminate primer-dimmers through the manipulation of melting temperatures.21
Performed of q (realtime-PCR): The q(rt-PCR) was performed by using AccuPower® Greenstar™ qPCR PreMix reagent kit (Bioneer, Korea) and Exicycler™ 96 Real-Time Quantitative Thermal Block (Bioneer, Korea), according to method described by Chen HF.22 The SYBER Green I based q(rt-PCR) premix reagent kit is designed for PCR amplification of cDNA for target gene by using LH-r primer and housekeeping gene (GAPDH).
Experimental Design of q (real time- PCR): For quantification of LH-r gene expression in dominant and cystic follicular cells, internal control gene as a housekeeping gene (GAPDH) was used for normalization of gene expression levels, therefore, preparing two q(rt-PCR) master mixes as the following premix.
Primer |
Sequences |
Product size |
GABDH- forward |
5'-AGCAACAGGGTGGTGGACCT-3' |
133 |
GABDH- reverse |
5'-AGTGTGGCGGAGATGGGGCA-3' |
|
LHR- forward |
5'-CCGGAAGGCGTCGTTGTGCAT-3' |
680 |
LHR- reverse |
5'-GCGTCGACCTCCGGGCCAT-3' |
Table 2 The Primers with their sequences and product size
qPCR premix |
Volume |
|
cDNA template |
10µL |
|
Primers |
LH-r -F |
2µL |
LH-r-R |
2µL |
|
DEPC water |
6µL |
|
Total |
20µL |
|
Table 3 Experimental Design of q (real time- PCR) for quantification of LH-r gene expression
qPCR premix |
Volume |
|
cDNA template |
10µL |
|
Primers |
GAPDH-F |
2µL |
GAPDH-R |
2µL |
|
DEPC water |
6µL |
|
Total |
20µL |
|
Table 4 Experimental Design of q (real time- PCR) for quantification of GAPDH-r gene
After that, q(rt-PCR) premix were added into AccuPower GreenStar q(rt-PCR) PreMixtube, then rt-PCR tubes sealed by the optical adhesive film and mixed by vigorous vortexing for re-suspension of premix pellet. The tubes centrifuged at 3000 rpm for two minutes by using vortex/centrifuge, then start Exicycler™ 96 Real-Time Quantitative, thermal Block instrument and performed optimization for target gens to determine the performance of SYBR Green I q(rt-PCR) assay, by identifying the optimal annealing temperature for each target gene, then loaded the specific Exicycler™ 96 Program to relative quantification, according to kit instruction as the following:
Step |
Condition |
Cycle |
|
|
95℃ for 5 min. |
1 |
|
Denaturation |
95℃ for 20 sec.℃ |
45 |
|
Gradient |
From 63℃, to 69℃, for 45 sec. |
||
Melting |
60℃ to 94℃ every 1℃, for 1 sec. |
1 |
Table 5 The steps, condition and cycles by Exicycler™ 96 systems which used for gradient temperature optimization
Statistical analysis
All the values are expressed as mean±Se. data of DF and COF results were analyzed using student t-test and appropriate p-values of less than 0.05 were considered as statistically significant.23
Samples
The ovaries which collected from local cows divided according in to macroscopic notation in to two groups, the DF group (n=23) with diameter 19.93±0.32mm. COF group (n=21) with diameter 37.56±0.64mm. Yet there was significant difference (P≤ 0.05) in the diameter COF group in comparison with DF group (Table 6).
Follicular state |
n. |
Follicular dim.(mm) |
E2 conc. In F.F.(ng/ml) |
P4 conc. In F.F.(ng/ml) |
DF |
23 |
19.93±0.32 |
314.39±2.55 |
50.25±1.57 E/P>1 |
COF |
21 |
37.56±0.64* |
865.96±10.64* |
84.308±1.35* E/P>1 |
Table 6 Differential between dominant and cystic ovarian follicles, data are presented as M±Se and t-test was used with (p≤0.05), (*) Significant differences
Estradiol-17β and progesterone assay in follicular fluid
The sex steroidal hormones concentration level in the FF of COF had higher E2 concentrations (865.96±10.64ng/ml), than did E2 concentrations in the FF of DF (314.39±2.55ng/ml), yet the P4 concentrations levels mean in COF showed higher (84.8±1.35ng/ml), compared to those in DF was (50.25±1.57ng/ml) (Table 6). There was a highly significant difference (P≤0.05) between two groups in E2 & P4 concentration.
Molecular analysis
Quantification of total RNA: The value of total RNA concentration was highly significant different (94.374±07ng/µl) in follicular cells mashed of DF, while in COF are 95.64 ±2.98.
cDNA Synthesis: All the total RNA samples were used in cDNA synthesis step by using AccuPower® RocktScript RT PreMix kit that provided from BIONEER company, Korea in reverse transcription reaction, for converted RNA to cDNA synthesis by using rt-PCR system (Excecycler 96)®. In temperature and time as in chapter three, this products reader by electrophoresis, then the cDNA bands were seen by U.V light, as Figure 1.
Quantitative real time-PCR: Data analysis of SYBR green I based rt-PCR assay were divided into primer efficiency estimation and relative quantification of LH-r gene expression level which normalized by housekeeping gene expression (GAPDH).
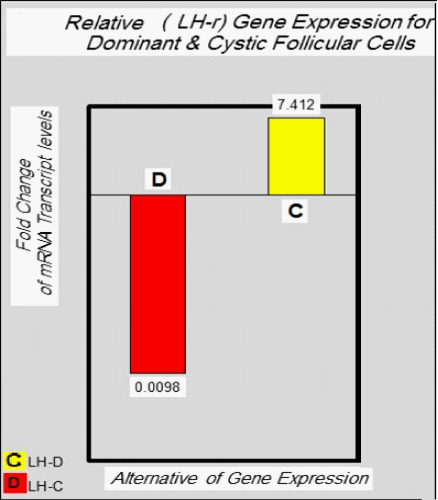
Relative Quantification of target gene expression: To calculate the relative expression of target gene in follicular cells of the DF & COF, the 2^ΔCtusing a Reference Gene Method used by normalizing target genes expression of LH-r gene are up-regulated (7.4127) in DF, and down-regulated (0.0098) in COF (Figure 2 & Figure 3) with expression of housekeeping or reference gene (GAPDH) Ct value = (20.1680) in DF, and (20.8869) in COF, (Figure 2 & Figure 4) (Table 7).



Samples |
Mean of CT values |
∆CT |
(2^ΔCT) Expression value |
|
GAPDH-r |
LH-r |
|||
Dominant Samples(n=14) |
20.168 |
17.2774 |
2.8906 |
7.4127 |
Cystic Samples(n=14) |
20.8869 |
27.559 |
-6.6721 |
0.0098 |
Table 7 The mean of Ct values and expression value of the LH-r gene in the follicular cells of dominant and cystic follicles
Normal expression ratio=7.4127/7.4127=1
Test expression ratio= 0.0098/7.4127= 0.0013 yield of this gene in follicular cells of COF(low expression or down−regulation)
Dominant and cystic follicular diameter
This study demonstrated that the high differential (p≤0.05) significant of follicular diameter size of COF (37.56±0.64mm) compared with DF (19.93±0.32mm) (Table 6). These result findings consider characteristic of cystic follicles by the presence of a high volume of follicular fluid, and agreements with more than one like Vanholder T18 and Youngquist RS.4
Estradiol & progesterone assay in follicular fluid
The follicular fluid of COF had higher E2 concentrations (865.96±10.64ng/ml) than follicular fluid of the DF (314.39±2.55ng/ml), yet the concentration of P4 was depressed (50.25±1.57ng/ml) in DF, in compared with the concentration of COF (84.308±1.35ng/ml) (Table 6).
The E2/P4 ratio was greater than one that’s indication to the DF came from ovaries in the follicular phase of the estrus cycle and hormonally classified as healthy (estrogen active) according to Mihm M et al.,27 also this hormonal results was complete agreement with many previous studies.7,8,25
Expression of LH-r Genes
The results of the present study confirms and extends the notion that there is a relative mRNA expression for the LH-r genes was highly down-regulated in follicular cells of COF alternative for mRNA expression of this genes in follicular cells of dominant follicles (Table 7) (Figure 3). which are high-regulated, these results agree with the observation of Ascoli M23 and Nogueira MF19 whose referring to increase of the LH-r in DF that remain estrogenic for prolonged period when exposed to low P4/high LH pulse frequency, furthermore this receptor concentrations in granulosa cells increased with follicle diameter.1 The study of Robert C24 showed that the increased of the number of LH-r protein in follicular cells, rapidly in the latter stage of antral follicular development furthermore the expression of LH-r protein increases as the follicles mature to become graafian follicles, therefore the granulosa cells of preovulatory follicles which have insufficient expression of LH-r as a result the ovaries were unable to fully respond to LH pulse, leading to a reduced rate of follicle rupture.
Calder MD et al.25 observed lower or even absent LH-r mRNA transcripts in theca and granulosa cells of COF, on the other hand Odore R et al.,26 observed similar receptor which have a high concentrations in COF and DF, Furthermore Mihm M et al.27 and Nogueira MF19 reported that the LH-receptor mRNA was about eight times higher in the dominant follicle, yet when compartment this results (highly down-regulated of the LH-r genes in COF) with previous studies which clearly established that the LH-r protein expression in the follicular cells was down-regulation of LH-r protein expression for prevent the ovary from repeated stimulation to pre-ovulatory surge of LH, which causes transient desensitization of LH response. This alteration may be the cause of COF28 which might be due to fact that the LH-r protein number reduced in follicular cells of COF, also Cook DL et al.,29 and Hamilton SA et al.,30 showed that the LH-r mRNA expression was numerically but not significantly increased in DF as compared with COF. This results of the present study may also explained the reduction rate of LH-r synthesis is not due to decrease transcription but rather to reduced LH-r mRNA half-life, these findings were disagreed with Calder MD et al.,25 who found that there are no significant differences in LH-r mRNA were observed between the dominant follicles and young cysts, but the LH-r mRNA expression increased in cystic cow, due to estrogen effect for prolonged periods when exposed to low P4/high LH pulse frequency as compared with dominant follicles. This high expression of granulosa LH-r mRNA may contribute to increase follicular steroidogenesis, while Lu DL et al.,31 claimed that the reasons for this discrepancy are not known and could be related to differences in tissue preparation and identification of healthy follicles, also the changes in the steady-state could result from either a decreased rate of receptors genes transcription and/or an increase in the rate of receptor mRNA degradation.
None.
Author declares that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.