Journal of
eISSN: 2374-6947


Review Article Volume 8 Issue 2
1Dr Kulvinder Kaur Centre for Human Reproduction, India
2Rotunda-A Centre for Human reproduction, India
3Swami Satyanand Hospital, India
Correspondence: Kulvinder Kochar Kaur, Scientific Director, Dr Kulvinder Kaur Centre for Human Reproduction, 721, G.T.B. Nagar, Jalandhar-144001, Punjab, India
Received: September 21, 2021 | Published: November 29, 2021
Citation: Kaur KK, Allahbadia G, Singh M. An update on role of mitochondrial transport in etiopathogenesis & management of various CNS diseases, neurodegenerative diseases, immunometabolic diseases, cancer, viral infections inclusive of COVID 19 disease-a systematic review. J Diabetes Metab Disord Control. 2021;8(2):91-103. DOI: 10.15406/jdmdc.2021.08.00228
Mitochondria represent complicated intra cellular organelles which classically have been isolated as the powerhouse of eukaryotic cells secondary to their key part in the bioenergetic metabolism. In more recent decades, the escalation of mitochondrial research has got invoked in researchers that have illustrated that these organelles are much greater than just simple powerhouse of cell, possessing the capacity of other crucial parts like signaling platforms which control cell metabolism, proliferation, and demise besides immunological reactions. In the form of crucial controllers, mitochondria on impairment are implicated, in the etiopathogenesis of a wide variety of metabolic neurodegenerative, immune in addition to neoplastic conditions. Much more recently, greater importance has been given by the researchers with the capacity they possess with regards to. Intercellular transfer which might implicate whole mitochondria, mitochondrial Genome or other mitochondrial constituents. The Intercellular transfer of mitochondria that by definition as horizontal mitochondrial transport can take place in mammalian cells both in vivo, as well as in vitro in addition to both physiological along with pathological situations. Mitochondrial shifting can yield an external mitochondrial source that can restore the normal mitochondrial replacing the mitochondria having undergone impairment, thus resulting in the enhancement of mitochondrial quality, or in case of tumor, altering their functional properties, in addition to chemotherapy responsiveness. Here we have tried to provide a comprehensive review with regards to the biological significance, modes beneath the event, besides their implication in various pathophysiological disorders, emphasizing its treatment potential with regards to diseases where mitochondrial impairment is the primary etiopathogenetic cause of the disease.
Keywords: intracellular mitochondrial transport, neurodegenerative disorders, tumors, immunometabolic disorders, tunneling nanotubes modulation, etiopathogenesis, mitochondrial whole vascular, disorders
mtDNA, mitochondrial DNA; ECV, extra cellular vesicles; NDG, neurodegenerative diseases; TNT, tunneling nanotubes; OXPHOS, oxidative phosphorylation; OS, oxidative stress; ROS, reactive oxygen species; GJC, gap junction channels; CNS, central nervous system; DS, Down syndrome
Mitochondria represent complicated intracellular organelles ,that have got isolated long back in the form of cellular power in view of their key part in oxidative energy metabolism.Working in the form of central metabolic hubs, mitochondria possess capacity to adapt to a variety of environmental hints besides metabolic changes meant for meeting the bioenergetic requirements of the cell that has got by definition for mitochondrial plasticity.1 In view of their markedly plastic quality mitochondria comprise a dynamic network constituted of signaling organelles that possess the capacity of a lot of functional crucial part in the cell proliferation, besides survival.2 Noticeably, a lot of preclinical in addition to clinical studies have illustrated that metabolic in addition to/or genetic, mitochondrial changes have implication with regards to etiopathogenesis of a lot of diseases that are inclusive of cancer.3 There exists marked intercommunication occurring with continuously occurring fusion in addition to fission processes.4 With the possession of capacity to dynamically remodel their morphological appearance besides keep themselves in motion within the cell, thus catering to their key energy power required.5 Mitochondrial pool homeostasis gets sustenance by selective depletion of mitochondria via mitophagy in addition to biogenetic mitochondria getting replaced, hence ensuring an active as well as dynamic mitochondria within the cell.6 Specifically, in neurons, antegrade mitochondrial transportation from the cell body to the axonal projections of cells can aid in the depletion of the damaged mitochondria with replacement of healthy mitochondria.7 In case of invasive cancer cells mitochondria readjust to the leading edge of the cell to yield fuel for their motion.8
The proof that has been coming out illustrates that the dynamic form of mitochondria might extend beyond cellular boundaries that aids in their translocation amongst mammalian cells, that radically questions the emergent beliefs with regards to intracellular separation of mitochondria along with mitochondrial DNA(mtDNA) getting inherited.9 Their part In signaling might further participate in intercellular cross talk, that demonstrated that mitochondrial genome in addition to the full mitochondria, both are mobile, thus can aid in delivery of the information amongst cells. This mobility of both mitochondria along with mtDNA has got named as ‘’momiome’’ to point that all mobility functions of mitochondria along with mitochondrial genome.10 The mitochondrial intercellular transport facilitates the integration of mitochondria into the endogenous mitochondrial network of the recipient cells, that aids In the alterations of the bioenergetic status in addition to other functional characteristics of the recipient cells ,both in vivo, along with in vitro.11 Moreover the horizontal transport of mitochondrial genes can result in robust influences on the pathophysiology of mitochondrial impairment.12 Despite the physiological significance of this event is still controversial, different in vivo along with in vitro studies have demonstrated how the shift of mitochondria in between cells possesses the capacity of recovering abnormalities in the recipient cells ,thus rescue in addition to controlling signaling proliferation, besides resistance to chemotherapy, in addition to working with regards to tissue revitalization.13 Earlier we reviewed with regards to Role of Mitochondrial Unfolding Protein Response in Reproduction and Aging with Utilization of Autologous Mitochondrial Injections in Mitochondrial Diseases along with in Aging Oocytes.14
The objective of this review was to update in context of scientific work with regards to the intercellular shift of mitochondria with concentration on the cellular along with molecular modes that modulate this event, detailing its biological significance in addition to implication, in case of variations in the pathophysiological relevance, that differs in immuno -metabolic diseases, neurodegenerative diseases/ neuro developmental conditions along with viral infections to cancer , besides emphasizing how targeting horizontal cell-cell transport of mitochondria can get utilized with regards to treatment of diseases that possess the properties of mitochondrial impairment.
Thus here we conducted a systematic review utilizing search engine Pubmed, google scholar; web of science; embase; Cochrane review library utilizing the MeSH terms like mitochondria transport /shift; tunneling nanotubes; extracellular vesicles (ECV); microvesicles ; gap junctions; cell fusion; expulsion of mitochondria; Bone marrow obtained stem cell; Neurodegenerative diseases (NDG); Alzheimer’s disease; Parkinson’s diseases; hippocampal neurons; astrocytes; Down Syndrome; Fragile X Syndrome; Extracellular mitochondria transport; acute central nervous system injury; immuno-metabolic diseases; obesity; metabolic Syndrome; cancer; in addition to viral infections Influenza; inclusive of SARSCoV2 Virus-COVID19 from 2000 to 2021 till date.
We found a total of 1950 articles out of which we selected 137 articles for this review. No meta-analysis was done.
The molecular modes via which cells that possess impairment of mitochondria manage to get new mitochondria from other cells in addition to signaling pathways controlling this event are still not clear. Probably cells have generated the modes that stimulate the shift in reaction to the damage signals that originate from the recipient cell. Nevertheless, we do not possess insight with regards to the molecular signals that start this kind of intercellular communication. Numerous structures are implicated in the transcellular mitochondrial transport.15 Major ones are i) tunneling nanotubes (TNTs) that depict the major cellular systems that modulate the cross talk with regards to transportation of mitochondria. Other mechanisms have got isolated, that are inclusive of membrane, microvesicles, gap junctions, cell fusion or expulsion of mitochondria16 Figure 1. Shift of mitochondria via these variations of structures can result in separate functional results for the recipient cells like acquiring functional mitochondria, immune activation or trans mitophagy.12 Thus a proper insight into the modes modulating mitochondrial shift would throw light on the mode by which this events get, controlled in addition to utilization of this can be done for therapeutic methods.
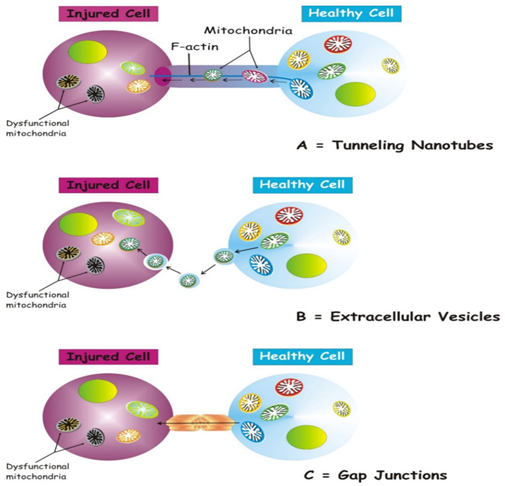
Figure 1 Courtesy ref no16-Three main forms of intercellular communication related to transcellular mitochondrial transfer. Under different injury signaling, three main forms of intercellular communication related to mitochondrial transcellular transfer can be formed: tunneling nanotubes (A) extracellular vesicles (B) gap junctions. (C) For details see the text.
Tunneling nanotubes (TNTs)
TNTs represent nanotubular structures which get developed via generation of cell membrane protrusions which stick to the target cells .The membrane of every cell move forward to fuse together, hence generating a firm ridge that besides getting connected get suspended In the extracellular space.16 TNTs aid in unidirectional as well as bidirectional transportation of separate types of constituents, inclusive of small molecules, proteins, organelles, besides viral particles as well.17 The invention of TNTs in 2004 ,seemed to be a novel cell- cell cross talk modes ,that illustrated the capacity of mammalian cells to inter change organelles with other cells.18 TNTs, work in the form of channels, besides being dynamic structures, possess the capacity of de novo generation, within a few minutes, possessing a half life that varied from a few minutes-various hrs.18 The TNTs possess a skeleton that comprises of F-actin in addition to transportation proteins which aid in the active shifting of constituents as well as mitochondria along these structures.19 TNTs work in the form of channels for the mitochondrial transport among different cells. Specifically, under conditions of oxidative stress (OS), the intracellular expression, of p53 gets unregulated with additional activation of the phosphatidyl inositide 3-kinase (PI3K) /protein kinase B (AKT)/ mammalian target of rapamycin inhibitors (mTOR) mTOR signaling pathway, resulting in the generation of TNTs among stressed in addition to unstressed cells modulating the transcellular shift of a lot of organelles, that is inclusive of endoplasmic reticulum (ER), golgi, endosomes, besides mitochondria.20 In the bone marrow microenvironment, in case of malignant melanoma, TNTs modulated transcellular shift of mitochondria from the adjacent non malignant bone marrow stromal cells to the multiple melanoma cells aids in oxidative phosphorylation (OXPHOS) of multiple melanoma cells as well as is based on the expression of CD38.21 TNTs further generate amongst tumor cells, besides getting implicated in their survival in addition to drug resistance.22
In case of neurons, the molecular motor myosin X is further needed for TNTs generation.23 In a study the part of the extracellular proteins, S100A4, took part in addition to its receptor advanced glycation end-products (RAGE) in management of TNTs, growth direction in the nervous tissue.24 During oxidative stress, hippocampal neurons in addition to astrocytes start the generation of TNTs, subsequent to p53 modulated activation of caspase 3. S100A4 cleavage via activated caspase 3 generates a gradient of low amounts of S100A4, in starting injured cells towards a greater amount in the recipient cells alias astrocytes.25 The outcomes of this study demonstrated that damaged neurons shift their cellular constituents to astrocytes that scatters the danger signals besides resulting in mitochondrial shift. TNTs, modulated intercellular mitochondrial transport can work in the form of a survival method for cells enduring stress like by salvaging injured cells by protection of the alveolar epithelial cells from damage,26 in addition to healing the tissues.27
Utilization of extra cellular vesicles (ECV) for mitochondrial transfer
A heterogenous population of vesicles (40-1000m in rage), usually get liberated from the intracellular to extracellular surroundings. These vesicles are termed extracellular vesicles (ECV),that are comprised of exosomes(30-100nm diameter), microvesicles (100 nm -1μm I dia), apoptotic bodies (1-2μm),on the basis of their source in addition to molecular structures.28,29 The apoptotic bodies have undergone minimal evaluation in view of their fast removal by the phagocytic cells.30 Exosomes along with microvesicles were initially given the definition of being vesicles meant for removal of ancient proteins in the immune cells, as well as reticulocytes,31 however recently it has been demonstrated that they are liberated from practically all cells , in addition to be believed to bring about cross talk in numerous pathophysiological conditions.32 ECVs that possess lipids, proteins, RNA in addition to mitochondria depict a relevant mechanism for the transportation of functional loads from one cell to the other.33 Pioneering of this has given us a new mechanism for crosstalk of an earlier existent communication means along with a new signal in transmission mode.34
Despite the mode via which mitochondrial proteins or mtDNA get picked up by ECVs remain questionable, though definitely, mitochondrial constituents have been found in ECVs. The smaller ones like exosomes, possess the capacity of transfer of RNA,35 however genomic DNA as well as mtDNA have further been found,36 larger ones like microvesicles possess the capacity of engulfing the full mitochondria.37 Microvesicles possessing mitochondria can get liberated by various cell kinds as visualized in immune cells, neurons in addition to astrocytes.11 This mitochondrial transfer is not restricted to injured cells, however act to get back organelles into other cells via a transcellular break down event.38 Moreover Morrison et al.,39 demonstrated, that mesenchymal stromal cells (MSCs), manipulated macrophages, causing enhancement of their respiration as well as phagocytic action in case of clinically significant lung damage models via ECVs that possess mitochondria.40 Significantly, these exosomes modulated transported mitochondria co-localize with the mitochondrial networks, besides generating reactive oxygen species (ROS), within the recipient cells.39
Transfer with the utilization of gap junction channels
Gap junction channels (GJC), represent a structure that connects the cytoplasm of 2 distinct cells. GJCs aid in passive transfer of nutrients, metabolites, 2nd messengers, cations, anions, besides mitochondria.27,41 Cx43 is a connexin that takes part in the transcellular shift of mitochondria. In a model of lipopolysaccharides (LPS)–stimulated acute lung damage, bone marrow stem cells (BMSC’S) shift mitochondria to the injured alveolar epithelium, an event that is dependent on Ca2+ exchange among the 2 cells via Cx43- GJCs.27 More recently it has been demonstrated, that mitochondrial shift from BMSC’S to haematopoietic stem cells (HSC’s) occurs as a timely physiological processes in the mammalian reaction to acute bacterial infection.40 Mechanism wise, the observation was that oxidative stress was controlling the opening of connexin channels that were PI3K-modulated system, whose activation aided the mitochondrial shift from BMSC’S to haematopoietic stem cells (HSC’s).39 Cx43- GJCs take part in the intercellular exchange of ROS,42 with a direct mitochondrial shift through Cx43- GJCs has got posited,16 that might depict a mode for generation of TNT in addition to intercellular transfer of mitochondria.
Other routes of Mitochondrial transport: mitochondrial expulsion as well as cell fusion
Besides the major routes of TNTs, ECV, Cx43- GJCs for the transcellular mitochondrial transport others are
Transcellular mitochondriar Shift in nervous cells
In case of the central nervous system (CNS), mitochondrial shift is constituted by a significant type of intercellular communication that aids in the homeostasis in addition to providing neuroprotection to the CNS.38 Mitochondrial shift in nervous cells takes part in case of cellular as well as tissue defense against the injury of the CNS, where they carry out pertinent functions with regards to damage.38 In case of CNS, astrocytes carry out numerous functions that are inclusive of neurogeneration, neuroprotection besides metabolism.12 Originating studies have demonstrated that astrocytes might represent the mode of mitochondrial shift from these cells to the injured neuron.38,48 In case of physiological situations, astrocytes protect neurons by counteraction of oxidative stress (OS), in addition to excitotoxicity, besides making sure of the neurotrophin provision.49 With regards to damage, astrocytes possess the capacity of shifting healthy mitochondria to axons.38,50 Cheng et al.,50 in a co culture comprising of human Induced pluripotent Stem Cells (iPSCs) carrying out numerous functions documented that astrocytes that were obtained from iPSCs had the capacity of shifting mitochondria to neurons, thus getting the dopaminergic neurons to get recovered from neuronal toxicity.50 This neuroprotection action of astrocytes obtained mitochondria shift to neurons has got emphasized in other studies of neurotoxicity, like, English et al.,51 illustrated in a study they conducted in vitro in case of a co culture of cisplatin-received neurons as well as astrocytes, like neurons which had obtained mitochondria from astrocytes, demonstrated, enhancement of neuron survival, got the reinvigoration of neuronal mitochondrial membrane potential in addition to made the calcium dynamics normal, that highlights the significance of transcellular mitochondrial shift.51 Noticeably, in case of stressful situations, astrocytes might stop their action with regards to neuroprotection as well as liberate danger signaling factors like proinflammatory cytokines, hence resultant injured neurons.12
Thus escalation of proof has documented that just like full CNS transcellular mitochondrial shift can possess significant importance with regards to a lot of functions in different pathophysiological conditions like i) Mitochondrial shift from neurons to astrocytes can result in an event known as transmitophagy, that aids cells to break down mitochondria with impairment.38 ii)the mitochondrial shift of astrocytic mitochondria to damaged neurons can result in advantages of neuroprotection.38,48 iii)active mitochondrial shift from endothelial progenitor cells, to neurons can result in enhancement of cell viability besides enhancing their actions to functional barriers.53 iv)This mitochondrial shift from HSCs as well as progenitor cells to neurons can result in enhancement of their mitochondrial functional effectiveness.14
Mitochondrial shift in impairment of mitochondria-associated neurodegenerative conditions: utilization of exogenous, mitochondria for treatment of Alzheimer’s as well as Parkinson’s diseases
The etiopathogenesis of neurodegenerative conditions like Alzheimer’s as well as Parkinson’s diseases, implicated impairment of mitochondria.54 Of the neurodegenerative diseases (NDD), Alzheimer’s disease (AD) is the commonest NDD, having the properties of continued depletion of neurons in forebrain area in addition to other brain regions.55 Impairment of mitochondria has been proven to be an early along with significant parameter of the disease.56 Numerous, besides opposite mechanisms implicate the impairment of mitochondria in case of AD pathophysiology in addition to their complicated regulation, makes the objective of targeting mitochondrial deficiencies markedly tough. With regards to, overcoming this restriction Nitzan et al.,57 made utilization of experimental method, that was tried with active in addition to functional mitochondria, thus letting mitochondria to work as full organelles instead of targeting only just one of their impairment tasks. The outcomes of this evaluation point that the action of shifting active intact mitochondria with the objective of effectively simulating mitochondrial function has advantages of treatment of AD action, that corrects the cognitive deficiencies, brain pathology, besides mitochondrial deficiencies in a mouse model of AD.57 In this, more recent in vivo study, the action of shifting active intact mitochondria was evaluated by treatment of AD mice(amyloid, intracerebro ventricular injection) intravenously with the freshly separated mitochondria.
Subsequently following 14days of mitochondrial transplantation, AD mice having treated with exogenous mitochondria demonstrated important enhancement of cognitive outcomes in contrast to nontreated control mice.58 An important enhancement of neuronal depletion in addition to reduction in gliosis were further observed in the hippocampus of treated mice in contrast to, nontreated AD mice.57 Escalation of mitochondrial action was further observed in the liver of mitochondria treated mice. No toxicity was seen to be correlated with the treatment. Thus mitochondrial shift might give an innovative therapeutic strategy with regards to treatment of AD. Parkinson’s diseases (PD) represents the 2nd commonest NDD, possessing the properties of selective depletion of dopaminergic neurons in the substantia nigra pars compacta (SNc) that possess motor in addition to non motor symptoms.58 The major histopathological (HPE), marker of PD is the existence in neurons of α-synclein (α-syn) protein collections generating inclusion bodies known as Lewis bodies.59 α-syn is basically is expressed presynaptically with proof of the presence of α-syn shifts from neurons to neuronal as well as non neuronal cells in vitro, that points to an α-syn pathophysiology promotes among anatomically close rain areas via an intercellular shift mechanism.60 Mitochondrial impairment is commonly appreciated as the common central pathway that influences the pathogenic event of sporadic as well as genetic PD.61 Impairment of mitochondria is a usual event in PD.62 Furthermore, α-syn can be existent at the mitochondrial membranes, with its collection can be correlated with mitochondrial impairment in PD.63 Escalation of ROS amounts that resulted in reduction of effectiveness in the electron transport chain (ETC) action are implicated in the generation of α-syn collection in addition to neuronal depletion.64
Escalation of proof pointed that astrocytes, possess a role significant in the propagation of PD.72 More recently, a definitive proof was revealed that the transneuronal mitophagy takes place in case of in vivo PD models.65 In these PD models astrocytes, are the ones initially implicated for the clearing of injured mitochondria, a functional part of marked significance with regards to PD that was correlated with mutations ,of Parkin as well as PINK1.61 Noticeably PINK1 action was mainly observed in astrocytes, whereas that was practically lacking in neurons.66 Exogenous delivery of mitochondria to injured areas might be a probable in addition to innovative treatment method for PD, as illustrated by Chang et al.,67 in his in vivo work that injection of mitochondria into median forebrain bundle (MFB) of 6 hydroxy dopamine unilaterally infused PD rats escalated the survival of dopaminergic neurons in addition to resulted in escalation of mitochondrial functions by aiding in the restoration of normal concentrations of mitochondrial complex I-IV in addition to causing reduction in oxidative stress in vivo.67 A greater need exists for the translation potential of mitochondrial transport for the treatment of AD as well as PD.
Mitochondrial transport neurodevelopment disease: extracellular mitochondrial liberation
Points to mitochondrial impairment in down syndrome as well as fragile X syndrome
Mitochondrial impairment is a key player aiding in the pathogenesis of robust neurodevelopment Diseases inclusive of Down Syndrome (DS), the commonest genetic deficiency resulting in intellectual incapacity that occurs secondary to trisomy of human chromosome 21. The properties of DS are Neuropathological alterations that take place in fetal as well as neonatal life resulting in aberrant brain generation.68 Aberrant mitochondrial bioenergetics negatively impact neuronal generation that is considered an early process in the generation of neurobiological changes that are typical of this Syndrome.69 Despite DS representing a multi–genic condition, which in a lot of ways, besides numerous signaling pathway are the observations seen being influenced, of which oxidative phosphorylation (OXPHOS), impairment was observed to be ubiquiously existent in any kind of tissue or cell kind, irrespective of age inclusive of fetal, so that DS is now believed to be an OXPHOS condition.70 More recently ,a new kind of extra cellular vesicles (ECV) that possess mitochondrial protein, known as mitovesicles were isolated in addition to observed to be changed in DS.71 D’Acunzo et al.,70 demonstrated that brain obtained mitochondria possess a particular subset of mitochondrial constituents, in addition to their amounts as well as cargo were aberrant in DS.70 A comparative evaluation of ECVs obtained from brains of Ts2 mouse model of DS as well as received from postmortem from human brains of person’s afflicted with DS illustrated greater concentrations of mitovesicles with aberrations constituents in the DS brain parenchyma, in case of murine as well as human postmortem brains.71 Figure 2a.These outcomes pointed that mitochondrial injury directly influenced mitochondrial biology, by activation of the liberation of these vesicles or via regulation of the mitochondrial constituents In a single ECV. In to these results demonstrate that mitochondrial concentrations, besides the constituents of mitovesicles ape the mitochondrial changes within the cells from they originated as well as their utilization as biomarkers for the evaluation of mitochondrial brain impairment, in case of neurological disorders.
Furthermore, mitochondrial brain impairment, aids in the pathogenesis of another robust Neurodevelopment Disease, namely Fragile X Syndrome (FXS). FXS further gets inherited that has the characteristics of mental retardation, that occurs secondary to the silencing of the fmr gene, that encodes the Fragile X mental retardation protein (FMRP),73 that represents an RNA binding protein that gets expressed basically in neurons in addition to astrocytes of the brain, besides correlated with about 4% of transcripts that were inclusive of mitochondrial proteins.74 Generation of neurons in case of Fmr1 1knock out (ko) mice illustrated dysfunctional maturation of dendrites changes in expression of mitochondrial genes, fragmentation of mitochondria, dysfunctional mitochondrial function in addition to escalation of oxidative stress.75 The 1st proof with regards to defective mitochondrial bioenergetics in the brain cortex of Fmr11knock-out (ko) mice, a model of FXS was demonstrated, by D’Antoni et al.,75 that validated the belief of dysfunctional mitochondrial possesses key part in the pathogenesis of FXS.76 More recently, capacity of ECV’s in shift of the mitochondrial constituents, in addition to their part in mitochondrial impairment was evaluated in case of astrocytes along with brain cortices from FLX model.72 The mitochondrial protein concentrations of the transcription factors nuclear respirator factors (NRF-1), along with ATPA ATPB of ATP synthase as well as mitochondrial membrane protein VDAC1 in ECV’s were observed to have a dramatic reduction in the cerebral cortex as well as astrocyte samples from Fmr1 1ko mice in contrast to euploid mice .These decreases are associated with a lot of mitochondrial biogeneration in the Fmr1 1ko fore brain that was correlated with reduction in the mitochondrial membrane potential in Fmr1 1ko astrocytes reduction was observed in the mitochondrial constituents of both ECV’s obtained from cerebral cortices as well as those liberated from astrocytes of the Fmr1 1ko mice Figure 2b.The loss of mitochondrial protein aids in the mitochondrial impairment of astrocytes.72
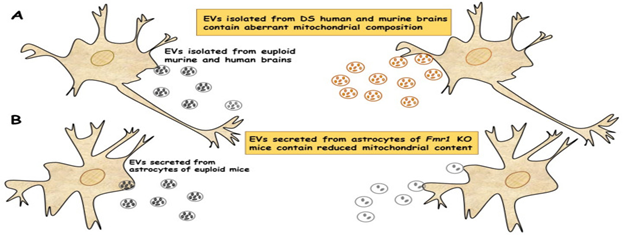
Figure 2 Courtesy ref no16-EVs of mitochondrial origin reflect mitochondrial dysfunction in DS and FXS. (A) Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in DS. Comparative analysis of EVs obtained from individuals with DS and Ts2 mouse model of DS showed higher numbers of mitovesicles with altered composition in the cerebral parenchyma, in both DS murine and human post-mortem brains.84 (B) Depletion of mitochondrial components from extracellular vesicles secreted from astrocytes in a mouse model of FXS. Mitochondrial components contained in EVs derived from both cerebral cortices and astrocytes of Fmr1 KO mice were found reduced.85
This study pointed that, the mitochondrial impairment of astrocytes is correlated with the pathogenesis of FXS, besides can do close monitoring in association with the loss of mitochondrial constituents. These observations might cause an escalation in the capacity of diagnosis of Neurodevelopment Disease that is correlated with mitochondrial impairment. Nevertheless, this type of study is developing in addition to future evaluation are required for getting insight for the exact mode implicated in the reduction in the mitochondrial protein that could simulate a deficiency in intracellular shifting from mitochondria to ECV’s, or in a compromise in ECV’s generation. Moreover, for getting better insight in the complicated physiological functions of astrocyte obtained ECV’s, it would be key to find the constituents of ECV’s, that cause key influence on the propagation of FXS Disease, besides its controlling mode.
Mitochondrial transport in case of tumor generation as well as resistance to chemotherapy
Cancer cells benefit from intercellular mitochondrial transport for taking care of the metabolic requirement, survival in addition to resistance to chemotherapy. The way Warburg posited, over 80 yrs back, that tumor cells have a classical inclination for upregulation of glycolysis.77 This action might appear to be contradictory, in view of glycolysis being from point of view of energy liberation not efficacious in contrast to OXPHOS, along with causes acidification of the extracellular microenvironment by escalation of lactate generation in addition to expulsion. Nevertheless, the metabolic switch towards glycolysis would be efficacious yield the intermediate blocks that are required for escalation of biogenerational requirements of the cancer cells. Despite maximum cancer cells favor glycolysis escalation of proof points that some solid tumors in addition to lot of haematological malignancies show normal or even escalated OXPHOS, along with mitochondrial metabolism.78 Actually, more recently, studies have suggested the significance of mitochondrial based metabolic reprogramming in escalation of cancer cells proliferation in addition to survival along with generation of resistance to chemotherapy in numerous cancer kinds.79 Cancer cell mitochondria have a key part in the crosstalk amongst neoplastic cells with the tumor surroundings.80 More recently, observations have suggested that tumors constitute besides malignant cells, instead they depict a complicated system of tumor as well as non tumor cells that generate a synergistic symbiotic association within the tumor microenvironment for ensuring survival in addition to resistance to chemotherapy.81 Cancer cells possess the capacity of expulsion of the whole mitochondria or certain of their cargo that is inclusive of mtDNA, cytochrome c, as well as formylated peptides, in the tumor microenvironment,82 that works as the damage–associated molecular patterns (DAMPs) which result in activation of the innate immune system.83 Despite activation of the hosts defense system by DAMPs, they further facilitate pathological proinflammatory along with immunosuppressive reactions which can result In cancer stimulation of cancer cell proliferation in addition to invasion.84
Mitochondria-based intercellular crosstalk among cancer along with non cancer cells via cell-cell contacts occurring,85 besides liberation of soluble factors in addition to ECVs,86 represents a crucial mode for cancer cells to get rid of immune scrutiny, besides generation of resistance to chemotherapy.79 Noticeably horizontal shift of mitochondria among cancer along with non cancer cells might possess a key part in the propagation of malignancy.21,87,88 Horizontal shift of mitochondria was initially illustrated by Spees et al.,84 in 2006. A 549 cells which were lacking mt DNA that got co-cultured with human mesenchymal stromal cells (MSC’s) or skin fibroblasts possessed the capacity of restoration of concentrations, besides a functional mitochondrial pool.85 Berrridge as well as Tan88 illustrated that in metastatic murine melanoma (B16) along with breast cancer (4T1) p0 cells possessed the capacity of restoration of functional mitochondria from the tumor microenvironment, hence effecting restoration of OXPHOS, besides tumor generation capacity in the parent cells.89 Furthermore, at a later date it got illustrated that mitochondrial transportation from MSC’s to p0 cells, resulted in recovery of mt DNA, OXPHOS, besides tumor generation capacity.87 In a classical horizontal shift of mitochondria, recipient cells possess the properties of escalated OXPHOS requirements along with robust reduction in mitochondrial function.21,90 whereas donor cells possess sufficient mitochondrial function in addition to being properly activated.88,91 Till now, just occasional molecular modulators that are implicated in horizontal shift of mitochondria have got isolated. Of these Matrix Metalloproteinase-1 (MMP 1), nestin in addition to proinflammatory cytokines are key modulators that are implicated in horizontal shift of mitochondria.21,90 Besides these factors PGC1α (that represent a master controller of mitochondrial bio generation) has been proposed to participate in shift of mitochondria from donor MSC’s to leukaemic cells.92 Moreover, donor cells activation is correlated with an escalation of ROS concentrations that are modulated by recipient cells,88,91,93 that points that ROS might be one of the modulators that are implicated in a directional shift of mitochondria. Robustly glycolytic cancer correlated fibroblasts (CAFs), that are believed to be involved in metabolic reprogramming of cancer cells,94 possess a tendency of donating their mitochondria to the adjacent prostate cancer cells, thereby resulting in stimulation of OXPHOS of cancer cells.95
These observations point that shift of mitochondria from CAFs is one more pathway that aids in the mitochondrial plasticity of cancer cells that might aid in tumor propagation. There is still altercation with regards to the importance of mitochondrial respiration in p0 tumor cells that result in stimulation of their tumor generation capacity. More recently, a study pointed that the basic cause is not “energy” or “greater ATP” requirements, rather, mitochondrial respiration would yield dihydroorotate dehydrogenase (DHODH), that is a necessary, intermediate with regards to, the de novo generation of pyrimidines.96 In agreement with this posit, loss of DHODH in tumor cells that possess capacity of totally functional OXPHOS resulted in repression of tumor generation, whereas repression of mitochondrial ATP synthase generated minimal action,96 hence pointed that DHODH is the probable treatment target in case of DHODH based cancers. Resistance to chemotherapy with regards to treatment of cancers still constitutes a key problem in evaluation with regards to effectiveness of treatment of cancers. A lot of studies have posited the influence of a lot of potential modes that are inclusive of intrinsic as well as extrinsic events, with the latter getting maximally influenced by intra tumor heterogeneity.97 Specifically, an important factor resulting in intratumor heterogeneity is the existence, in the microenvironment of numerous non malignant cells that get recruited in the tumor area like CAFs, MSC’s, in addition to immune cells.98 Intercellular communications as well as crosstalk, amongst malignant in addition to non malignant cells are key in case of intratumor heterogeneity besides resistance to chemotherapy.97 Like MSC’s, obtained from Bone marrow specimens of patients with acute lymphoblastic leukaemia (ALL) transformed to an active cancer- correlated fibroblast phenotype on treatment with the chemotherapeutic drugs cytarabine as well as daunorubicine in addition to avoided treatment stimulated apoptosis of ALL cells by shift of mitochondria via TNTs.99 The Intercellular mitochondrial shift is an interesting mode where insight is still partly clear, whose targeting might yield opportunities in cancer treatment. Moreover, proof with regards to mitochondrial shift might take place akin in solid as well as haematological tumor cells further escalate the significance of this event. Moreover, the influence of mitochondrial shift at the time of tumor propagation along with generation of resistance to chemotherapy might reason out the mode that is still ill understood of the effects of some anti cancer drugs.
More recently, proof pointed to a part of intercellular mitochondrial transfer in the control of the immune system.100 Court et al.,99 documented that the amalgamation of exogenous mitochondria facilitates the programming of regulatory T cells in the steady state that pointed that mitochondrial transfer might possess anti-inflammatory characteristics.99
Mitochondrial transfer from MSC’s to macrophages as well as T cells
At the time of tissue healing, macrophages play as a central actor in the removal of inflammatory constitutents via phagocytosis. Macrophages represent cells in the immune system which possess a key part in the inflammatory reaction, that have the properties of a wide spectrum of separate polarization as well as activation states, whose extremes are the ‘’classically activated’’ along with, proinflammatory macrophages M1 as well as the alternatively activated’’ in addition to as well as anti-inflammatory M2 macrophages. MSC’s possess capacity of escalation of the anti-inflammatory activity of macrophages by stimulation of their differentiation to M2 phenotypes.101,102 Various in vivo, in addition to in vitro studies have demonstrated, that mitochondria get transferred from MSC’s to macrophages by stimulation of selective differentiation to M2 phenotypes thus aiding in the antimicrobial actions of MSC’s.40,103 In case of acute respiratory environmental distress syndrome, OXPHOS action as well as phagocytosis of macrophages was stimulated subsequent to acquisition of mitochondria from macrophages,103 with this posit given that stimulated OXPHOS were implicated for the transformation, of the M2 phenotype of macrophages.47 Thus in turn hampering the intercellular mitochondrial transfer by influencing the mitochondria of MSC’s,47 or by blockade of this transfer pathway,103 avoids phagocytosis, in addition to, bioenergetics of macrophages.
Besides macrophages, pathogenic T helper (Th17) cells can further pickup mitochondria from bone marrow obtained MSC’s in a co-culture system that escalated utilization of oxygen in turn as well as resulted in reduction of IL17 generation by Th17 cells.104 Moreover bad mitochondrial shift to Th17 cells from the synovial stromal stem cells was seen in patients of rheumatoid arthritis in contrast to bone marrow MSC’s that got identified from healthy donors.104 More recently, the action of intercellular mitochondrial transfer as well as direct transplantation of MSC’s obtained mitochondria to the peripheral blood lymphoid , mononuclear cells was evaluated.100 Mitochondria obtained from transplanted MSC’s have got illustrated to be attained inside cells with escalation of expression of mRNA that were correlated with activation of T lymphocytes, as well as Treg differentiation of regulatory T cells (Treg) (FOXP3, IL2RA, CTLA4, as well as TGFβ1 [transforming growth factor beta])), resulting in escalation of number of Treg cells as well as as a subsequent immunosuppressive reaction.100 Hence intercellular mitochondrial transfer might be an innovative target for MSC’s which can promote immunoresponse in addition to aid in treatment of diseases,
Mitochondrial transfer from adipocytes to macrophages as immune-metabolic interaction controlling metabolic homeostasis dysfunction in obesity
Metabolic inter action amongst adipocytes as well as immune cells are necessary for ensuring tissue homeostasis, besides an impairment, can result in pathological inflammation which results in originating obesity in addition to obesity correlated metabolic impairment.105–107 Intercellular mitochondrial transfer occurs in vivo, in the white adipose tissue(WAT) with the axis of mitochondrial shift from adipocytes to macrophages that is observed to be impaired in obesity.125 Brestoff etal.,108 further demonstrated the effectiveness of this event in case of nutritional disorders like diet induced obesity (DIO) secondary to the reduction in consumption of mitochondria by the WAT possessed by them.108 Figure 3 Macrophages which have gained mitochondria from adipocytes tend to generate excessive ROS, besides illustration of hypoxia signals along with de-enrichment of genes that are encoded by nucleus that are implicated in the mitochondrial homeostasis in addition to the sustenance of ETC.108 In contrast to that the mitochondrial shift from adipocytes to macrophages is significantly decreased in obesity, that is a proinflammatory condition, where macrophages get exposure to factors which get stimulated by a type 1 immune reaction (M1).106,109,110 The directional mitochondrial shift to macrophages takes place commonly in healthy WAT when macrophages are predisposed to a M2 activation state that get stimulated by type2 cytokines like IL-4 as well as IL-13 that get generated by innate group 2 lymphoid cells, eosinophils, besides other cell kinds, existent in healthy WAT.106,107 This pointed that, M1 -like polarization hampers mitochondrial entry. This implicated that decrease in mitochondrial shift is a characteristic of metabolic disease like obesity, in case of WAT at least.

Figure 3 Courtesy ref no16-Intercellular mitochondrial transfer from adipocytes to macrophages. WAT-resident macrophages take up mitochondria from neighboring adipocytes in a heparan sulfate-dependent process that is impaired in obesity. Genetic disruption of mitochondria uptake by macrophages reduces energy expenditure and aggravates diet-induced obesity in mice, indicating that intercellular mitochondria transfer to macrophages promotes systemic metabolic homeostasis.
The modes implicated in mitochondrial shift in to cells are not clear right now. The mitochondrial uptake gets hampered by cytochalasins which hamper the remodeling of the actin cytoskeleton.106,111 Via genomic screening, Brestoff et al.,108 isolated 23 candidate genes which might aid in mitochondrial uptake. Of these, 13 genes are implicated in the HS biogeneration of heparan sulfate (HS) pathway.108 Intercellular mitochondrial transfer takes place minimally partly via a HS-based pathway in vivo in addition to, in vitro, besides removal of genes might be implicated in intercellular mitochondrial transfer to macrophages, besides resulting in changes in energy homeostasis, hence pointed that the probability of a functional association. Actually patients as well as mice that possessed functional correlation with no loss of function mutations in Ext1 (exostosin 1) that encodes the copolymerase implicated in HS biogeneration,106 that presents as homeostasis dysfunction with regards to lipid in addition to glucose homeostasis.113 Intercellular mitochondrial transfer to macrophages might take part in the sustenance of energy homeostasis in mice. These observations just validate further with regards to obesity correlated reduction in mitochondrial shift from adipocytes to macrophages might have a role in weight accretion. These outcomes pointed that a novel new manner of immuno-metabolic interaction amongst cells where in certain cells like adipocytes transport their mitochondria to macrophages for regulation of systemic metabolic homeostasis. A new treatment approach might target the intercellular mitochondrial transfer as a novel method for therapy method of metabolic diseases management.
Extracellular mitochondria might be existent in the extracellular space as well in intact as well as free form (freeMitos) or get surrounded by a membrane, like with in platelets, or vesicles, or in the form of free circulating mtDNA (ccf mtDNA), with separate cells reveal a new arena of study in which mitochondria work further from their parts in the form of cell’s power houses, here acting here as signaling organelles.9,114 Possessing the knowledge of the part of extracellular mitochondria, besides their separate form might prefer the generation of novel treatment strategies for escalation, of health, besides isolation of new biomarkers of diseases.
Extracellular circulating cell free mitochondria in blood
Various indications have illustrated the existence of circulating cell free circulating mitochondria in the blood that get liberated from various cell kinds in situations of stress, damage, or disease.114 More recently, Stephens et al.,115 demonstrated cell free mitochondria liberate with regards to, non pathological conditions, besides their estimation in the form of circulating mitochondria in murine as well as human blood.115 Stephens et al.,115 utilized flow cytometry for the estimation of circulating mitochondria in platelets, depleted plasma in case of healthy mice in addition to humans, that illustrated that circulating cell free mitochondria possess an active transmembrane potential, besides possesing the capacity of getting in the p0 cells. With the utilization of a proteomic study recall of mitochondria particularly in addition to ECV- correlated proteins in sorted circulating cell free human mitochondria occurred.115 These observations were agreeable with a recent article that demonstrated the existence of cell free mitochondria revealing normal oxygen utilization by the blood of healthy human subjects.116 Dache et al.,116 gave an assessment of 200,000-37,000,000 respiration-competent mitochondria /ml of extracted plasma, which pointed that the circulating cell free mitochondria respiration-competent mitochondria might depict an innovative class of signaling organelles that are implicated in controlling action, besides intercellular crosstalk. Despite, the proof for the existence of cell free mitochondria in human blood is quite impressionable, the conclusions of these mitochondria being energy competent/ functional with regards to respiration has been disputed.117 Steir117 tried to conduct an assessment of functionality with regards to respiration in human blood with the utilization of high resolution respirometry in addition to platelets extracted mitochondria from the same blood samples in the form of positive controls.
Despite, the existence of cell free mitochondria in human plasma, no proof that validated that mitochondrial electron transport system (ETS) was functioning, as illustrated via assessment of factors like respiratory rate whose observation was not significantly variable from 0, besides no significant reaction to ADP along with absence of sensibility to uncoupler as well as /or OXPHOS inhibitors. Nevertheless, the action of the complicated IV in vitro was estimable besides being minimally greater in contrast to the concentrations seen in mitochondria extracted from platelets, which pointed that cell free mitochondria in human blood possess the capacity of retaining just a non functional role of ETS. Despite, the existence of doubt with regards to them functionally possessing the capacity of OXPHOS action, circulating mitochondria might possess significant physiological parts that are required to get explained. Trying to find the characteristics, of these circulating mitochondria is significant for definition of baseline in healthy persons which will aid in contrasting in pathological situations. Giving definition of origin in addition to functions of extracellular function mitochondria would be key for gathering insight with regards to full implication in health as well as disease.
Extracellular cell free circulating mitochondrial DNA in form of danger signal
Mitochondria represent the sole intracellular organelles whose existence, is outside the nucleus, which possess, their own genome, namely the mitochondrial DNA. In mammals mt DNA represents the intron less circular double-stranded molecule that Is maternally inherited, besides exist in numerous copies/cell.118 The mitochondrial genome is composed of 37 genes-13 of which encode protein subunits of the OXPHOS, machinery that generates ATP, 2rRNA, (12S as well as 16S) along with a total sets of 22 tRNAs for mitochondrial protein generation.119 The absence of an effective DNA healing systems in addition to protection conferring histones that exist in the nuclear DNA allows greater proneness of mt DNA to mutations.120 Physical stressors like injury, infection, robust exercise can facilitate mt DNA expulsion from cytosol to the microenvironment, till it arrives in the circulation as cell free mt DNA (ccf mt DNA).121 In view of the bacterial origination, of these mt DNA, ccf mt DNA is still acceptable, in the body as foreign along with as a consequence it initiates an immunogenic resulting in an inflammatory reaction.122 On liberation by the lymphocytes, ccf mt DNA stimulates immune activation123 pointing to the presence of cell kind–based modes for controlling the mt DNA liberation, mt DNA can get expelled from mitochondria which causes collection of the injured components, that works as a stimulus for the immune cells, resulting in either stimulation of proinflammatory signals124 or aid in recombination, besides mixture with the recipient cells genome on transportation within the ECV’s.9,125
Inflammation induction secondary to mt DNA subsequent to robust injury in patients, manifestation of multiorgan failure results in escalation of concentrations of ccf mt DNA.126 Additionally, escalated ccf mt DNA observed in patients, of DM, cancers as well as,124 myocardial infarction (MI) that points to ccf mt DNA a poor prognostic markers in these pathological disorders. ccf mt DNA is usually believed to be an alarming signal, that works like the damage–associated molecular patterns (DAMP) molecules.15,82 The signals developed by mitochondrial obtained DAMPs might be chemotactic, phagocytic, immune activating or resulting in regeneration. Like mt DNA DAMPs possess the capacity of stimulation of neutrophils, hence activation of extra immune cells which result in liberation of proinflammatory cytokines, which can further cause progression of injury to far off tissues/organs.127 Further, more insight of ccf mt DNA as well as ccf mt DNA DAMPs might escalate our knowledge with regards to inflammatory reaction in addition to might yield in the end innovative molecular strategies for generation of anti inflammatory treatments.
Lots of studies have illustrated that several viral infections might change the mitochondrial function in addition to the cellular innate immune system reaction for sustenance of intracellular survival.128 Viruses possess the capacity of modulation of cell energy, reprogramming the metabolic pathways, besides utilization of metabolites for the maintenance of viral niches in the cells.128 Intriguingly, TNT’s have been illustrated in facilitation of spreading of viral infections in various steps with regards to the cycle of infection.129 Numerous viruses inclusive of the flu virus, the HIV virus, herpes simplex virus, utilize TNT’s for the shifting of their genetic material with in the target cells, that is followed by the modulation of mitochondrial functioning capacity.129 Thus viruses attain capacity of evasion of host immune system, besides bypassing the pharmaceutical targeting with the objective of avoidance of their entry into the cells via the plasma membrane receptors.17,130 Like human macrophages, that are existent in lymph nodes obtained from HIV infected individuals showing HIV getting re activated demonstrated TNT’s like structures.131 TNT’s modulated spreading of viral infection might escalate in addition to cause exaggeration of the viral disease by targeting numerous organs as well as tissues , the way it has been detailed for SARSCoV2 Corona virus modulated COVID-19 disease. The complicated ` pathological situations, secondary to the pandemic SARSCoV2 Corona virus infection displays a wide spectrum of, clinical manifestation resulting in generation of acute respiratory syndrome that is associated with the cytokines storm in addition to multiorgan failure in cases of robust cases.132 Despite, the mode that results in robust COVID-19 disease is continuing to be not understood, there are chances that an escalation of innate immune reaction worsening might occur.133
The proof that is emergent is that, SARSCoV2 hijacks mitochondria of the immune cells with reproducibility within the mitochondrial structures, that causes impairment of the dynamics of the mitochondria eventually resulting in cell death.134 SARSCoV2 might further cause manipulation of the mitochondrial function, directly in addition to indirectly. SARSCoV2 gains entry in the host cells via getting attached to the angiotensin converting enzyme 2 (ACE2) that is followed by internalization along with loss of ACE2 receptors.135 In case of vascular endothelium, ACE2, transforms angiotensin II to angiotensin. Hence ACE2 deletion is resulting in escalation of angiotensin II, that is a prothrombotic, vasoconstrictive along with proinflammatory peptide hormone which enhances ACE2 as well as cytoplasmic in addition to mitochondrial ROS concentrations, resulting in Oxidative stress, along with mitochondrial impairment.136 The indirect action on mitochondria result in facilitation of acute lung damage usually seen in robust cases of COVID-19 infection. Other than this indirect action SARSCoV2 might directly cause manipulation of the mitochondrial function via the accessory proteins, Orf9b, that represses interferon I (IFN1) reactions by binding to target TOM70, that is an outer membrane mitochondrial protein.137 Activation of IFN1 is a key action of the immune defense reaction against the viral infection,138 hence its repression would cause facilitation of virus replication as well as COVID-19 disease.
Additionally, more recently a study pointed that SARSCoV2 represses mitophagy,139 hence result in facilitation of collection of injured mitochondria which inflammation along with cell demise.136 Viral Orf s, that work on host mitochondria, can facilitate the liberation of mtDNA in the cytoplasm, hence activation of mtDNA–stimulated inflammasome as well as repression of innate as well as adaptive immunity.136 Furthermore, evaluation that has the objective of finding how viruses crosstalk with host mitochondria might yield further insight with regards to COVID-19 disease pathology besides innovative treatment approaches. The part of mitochondria at the time of hyperinflammatory state alias ‘’cytokine storm’’, in COVID-19 still needs clarification. More recently, a study posited that ’cytokine storm ‘modulated iron impairment, result in ROS generation along with oxidative stress as well as phenotypically seeming to be hyperferritinemia, that was associated with the robustness of disease.140 Thus Saleh et al.,140 pointed that the inflammatory signals stimulate a vicious cycle that further causes deterioration of mitochondrial oxidative injury along with aids in coagulopathy as well as ferroptosis.140 Moreover, Saleh et al.,140 further pointed that COVID-19 infections besides causing intracellular mitochondrial impairment, further influenced, the extracellular mitochondria, specifically platelets mitochondria that result in facilitation of blood clots as well as thrombosis. These extracellular mitochondria might work as treatment target to signal the robustness of COVID-19 disease.140 Besides, extracellular whole mitochondria, significant escalation of circulating mitochondrial DNA have been estimated in the blood of COVID-19 patients with robust clinical presentation of the disease,141 disclosing emergent markers for COVID-19 treatment. Figure 4 shows how mitochondria are involved in COVID-19 pathogenesis.

Figure 4 Courtesy ref no16-Involvements of mitochondria in COVID-19 pathogenesis. Schematic illustration showing the SARS-CoV-2 entry into the host cell by attaching to angiotensin-converting enzyme carboxypeptidase 2 (ACE2). Once inside the cell, viral RNA and proteins localize on mitochondria, resulting in ACE2 depletion and increase in Angiotensin-II, which, in turn, mediates mitochondrial dysfunction (more details in the text). SARS-2-CoV-2 targets the mitochondrial machinery also directly through one of its encoded proteins, Orf9, which promotes release of mtDNA and further mitochondrial dysfunction, leading to enhanced inflammasome activation.
More recently, experimental proof with regards to the mitochondria possessing the capacity of interaction besides the ability to move further than cell boundaries, in a lot of pathophysiological conditions, queries the earlier prototype of intracellular separation as well as how mtDNA get inherited, that opens a new pandoras box with the invention of a greater cross connective dynamic in addition to plastic kind of mitochondrial biology. Detection of the molecular modes in addition to signaling pathway resulting in control of intercellular mitochondrial transfer would be of utility for facilitation of its probable treatment utilization. In this aspect ,future treatment approaches need to get generated for the enhancement of cell- cell transport of organelles when of functional utility or its avoidance when harmful, like at the time of spread of viral infections, besides keeping neoplastic cells to be kept in containment as well as avoidance of generation of resistance to chemotherapy. A new mitochondria dependent treatment strategy, that is dependent on direct or systemic delivery of mitochondria from autologous sources has got generated.142 This kind of treatment with regards to getting the mitochondria that are impaired getting replaced resulting in escalation of cellular bioenergetic as well as reduction in oxidative stress.143 Supplementation of healthy mitochondria from the same patient might result in further advantageous results.144
Mitochondrial transplantation approaches yield advantageous results for the treatment of cardiac ischaemia, neurodegenerative disease as well as reperfusion of liver,145 though the factors implicated in intercellular mitochondrial transfer need characterization. In trying to explain a mitochondrial transport / transport dependent approaches, a requirement is to take into account that the mtDNA in addition to mitochondrial constitutents are markedly immunogenic in view of their bacterial initiation. Furthermore, the significant part of mitochondria has to be considered with regards to any patho physiological disorders, in addition to carefully review if the mitochondrial transport aids in, facilitation of health or diseases in various clinical settings.
Here a comprehensive insight with regards to the intercellular mitochondrial trafficking with details of modes beneath this event as well as implications in various diseases. The way emphasis has been laid here the cell-cell transport of mitochondria depicts a widespread process implicated in various pathophysiological settings, although the precise molecular modes that modulate intercellular mitochondrial transport as well as signaling controlling the event is still not clear. More studies are required for finding what stimulates mitochondrial transport, the mode of generation of the network of intercellular bridges in addition to communication in several transfer models as well as their probable utilization in separate clinical settings.
None.
The authors declare that they have no conflicts of interest.
None.

©2021 Kaur, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.