Journal of
eISSN: 2373-4396


Background: ST segment elevation and deviation in lead aVR can provide useful prognostic information in patients with ST elevation myocardial infarction (STEMI). The aim of the present study was evaluation of the effect of aVR ST elevation (aVR-STE) and aVR ST deviation (aVR-STD) the in-hospital and six-month prognosis of patients with STEMI.
Methods: The study was a cohort of medically treated patients with acute STEMI. The patients were categorized as aVR ST elevation (aVR-STE) or aVR ST deviation (aVR-STD) if there was > 0.05 mv ST elevation or ST deviation in lead aVR, respectively; otherwise, they were categorized as control groups.
Results: 334 patients [49 patients (14.67%) with aVR-STE and 159 patients (47.60%) with aVR-STD] were included. The mean age of the study group was 59.62 ± 13.03 years and 75.4% were male. In-hospital mortality was not significantly different in patients with or without aVR-STE or aVR-STD. Pulmonary edema or overt decompensated heart failure was seen more in patients with aVR-STE [Relative risk (RR): 3.393, 95% confidence interval: 1.405-8.192, P = 0.012] and it trended more in patients with aVR-STD (P = 0.06).
6-month follow up: Mortality was not significantly different among patients with or without aVR-STE or aVR-STD. Pulmonary edema or overt decompensated heart failure occurred more in patients with aVR-STE and aVR-STD (RR: 3.190, 95% confidence interval: 1.684-6.046, P < 0.0001, RR: 1.937, 95% confidence interval: 0.987-3.800, P = 0.049).
Conclusion: ST elevation or deviation in lead aVR was not an indicator of in-hospital and six-month mortality in our study. However, its effect on pulmonary edema or overt decompensated heart failure requires more study.
Keywords: ST elevation myocardial infarction; Electrocardiography; Prognosis; ST segment elevation in lead aVR
CKMB: Creatine Phosphokinase type MB; RR: Relative Risks; aVR-STD: aVR ST Deviation; aVR STE: aVR ST Elevation; CAD: Coronary Artery Disease; CKMB: Creatine Phosphokinase type MB; DBP: Diastolic Blood Pressure; HDL: High Density Lipoprotein Cholesterol; LDL: Low Density Lipoprotein Cholesterol; LV: Left Ventricle; SBP: Systolic Blood Pressure; STEMI: ST Elevation Myocardial Infarction; VS: Versus; PE-HF: Pulmonary Edema or overt decompensated Heart Failure; RI-CP: Recurrent Ischemic Chest Pain
Lead aVR is often ignored in standard 12-lead ECG [1-3], as it is oriented to the upper-right side of the heart and is not adjacent to other leads. Hence, ST-segment elevation in aVR has been associated with severe coronary artery lesions in patients with STEMI [4-7] and other acute coronary syndromes [6,8-12] and may be an indicator of more important coronary occlusion [4-7,13-19] ST segment changes in lead aVR may also be correlated with left ventricular function, mortality and prognostic findings during the hospital stay of patients with STEMI [1,4,14,15,18,20,21], and UA/NSTEMI [2,3,10]. Nonetheless, the prognostic value of ST elevation and ST deviation in lead aVR-particularly in longer follow up-requires more study. Thus, we decided to study the in-hospital and six-month prognostic value of aVR ST elevation (aVR-STE) and aVR ST deviation (aVR-STD) in STEMI.
The study was a cohort of patients with acute STEMI (started at April 2010 and finished at December 2012) in two hospitals with CCU in Shahroud, Iran, which were the only centres with CCU in the township. Patients with ST elevation (STEMI) were included if they presented within 12 hours of symptom-onset. Excluded patients were those with a left bundle branch block, left ventricular hypertrophy, ventricular paced rhythm at presentation, and those who did not sign the written informed consent. There was no facility for primary PCI in the city, and patients were treated medically according to the present guidelines, and fibrinolytic therapy with streptokinase was prescribed unless contraindicated. The initial 12-lead ECG was obtained at the emergency room at the time of admission. Heart rate, systolic and diastolic blood pressure and creatine phosphokinase type MB (CKMB) were also measured at the admition time. A single investigator blinded to clinical data examined all the ECGs. ST elevation in lead aVR was defined as > 0.05 mV of ST elevation in lead aVR, 20 ms after the J point, using the preceding TP segment as the baseline. ST deviation in lead aVR was defined as deviation of the ST segment, including ST elevation > 0.05 mv, 20 ms after the J point or ST depression > 0.05 mv 80 ms after J point.
Transthoracic 2D and Doppler echocardiography were performed for all of the patients within three days of admission and ejection fraction was detected by the eyeball method. All of the included patients were followed up during admission time and 316 patients completed six-month follow up. Our primary endpoint was a comparison of mortality during in-hospital admission among patients with and without aVR-STE. The secondary endpoint was a combination of any of the following adverse events during admission and six-month follow up:
For the statistical analysis, the statistical software SPSS version 16.0 for windows (SPSS Inc., Chicago, Illinois) was used. Numerical variables are presented as mean ± SD, and categorical variables are summarized by raw numbers and percentages. Continuous variables were compared using the Student's t-test or the nonparametric Mann-Whitney U test whenever the data did not appear to have a normal distribution, and categorical variables were compared using the chi-square or Fisher's exact test, as required. Relative risks (RR) and 95% confidence intervals were calculated if needed. Multivariate analysis using logistic regression or Cox regression analysis with the backward Wald method was used whenever needed and Kaplan-Meier curves were drown.
Sample size calculation: Based on the assumption that the baseline incidence of in-hospital death in patients with aVR-STE would be 19% and 5% in the those without aVR-STE, and that the incidence of aVR-STE would be about 16% [14], with an α error of 0.05, a β error of 0.2 and a power of 80%, around 47 patients in the aVR-STE group and 282 patients without aVR-STE were needed.
In total, 334 patients [49 patients (14.67%) with aVR-STE] were included in the study. There were 110 patients (32.93%) with aVR ST depression, and hence 159 patients (47.60%) had aVR-STD. The mean age of the study group was 59.62 ± 13.03 years and 252 patients (75.4%) were male. The basal characteristics of the studied groups were almost uniform spatially regarding drug therapy including treatment with fibrinolythics in aVR-STE and aVR-STD groups in comparison with controls. Important basal characteristics and those with significant difference are presented in Table 1. In patients with aVR ST depression, the involvement of the inferior wall was seen in 64 patients (58.18%) and the anterior wall and the lateral wall in 24 (21.82%) and 23 (20.91%), respectively.
Variable |
aVR-STE |
Without aVR-STE n=285 |
P value |
aVR-STD n=159 |
Without aVR-STD n=175 |
P value |
Age(year) |
59.95 ± 13.01 |
59.56 ± 13.05 |
0.845 |
60.6 ± 12.7 |
58.6 ± 13.2 |
0.168 |
Sex (male) |
35 (71.4%) |
217 (76.1%) |
0.479 |
121 (76.1%) |
131 (74.9%) |
0.792 |
Hyperlipidemia |
13 (26.5%) |
42 (14.7%) |
0.04 |
24 (15.1%) |
31 (17.7%) |
0.519 |
Family History of Premature CAD |
14 (29.2%) |
54 (19.4%) |
0.125 |
41 (26.3%) |
27 (15.9%) |
0.021 |
Summed ST Elevation |
13.64 ± 10.99 |
11.01 ± 9.56 |
0.092 |
13.63 ± 10.99 |
9.3 ± 8.09 |
<0.001 |
Summed ST Deviation |
18.46 ± 11.24 |
14.27 ± 10.42 |
0.002 |
18.44 ± 11.72 |
11.66 ± 8.34 |
<0.001 |
Anterior Wall STEMI |
42 (85.7%) |
154 (54%) |
0.0001 |
86 (54.1%) |
110 (62.9%) |
0.104 |
Inferior Wall STEMI |
6 (12.2%) |
127 (44.6%) |
0.0001 |
70 (44%) |
63 (36%) |
0.135 |
Lateral Wall STEMI |
3 (6.1%) |
32 (11.2%) |
0.281 |
26 (16.4%) |
9 (5.1%) |
0.001 |
Previous Therapy with Statins |
5 (10.2%) |
14 (4.9%) |
0.175 |
7 (4.4%) |
12 (6.9%) |
0.321 |
CKMB (IU/L) |
86.19 ± 113.5 |
112.5 ± 173.8 |
0.722 |
102.4 ± 144.6 |
114.5 ± 184.5 |
0.621 |
LDL (mg/dL) |
93.1 ± 27.3 |
103.9 ± 30.2 |
0.023 |
98.1 ± 26.3 |
106.2 ± 32.6 |
0.016 |
HDL (mg/dL) |
37.9 ± 9.5 |
41.8 ± 11.6 |
0.045 |
40.4 ± 10.1 |
42 ± 12.4 |
0.308 |
Cholesterol (mg/dL) |
163.4 ± 40 |
179.9 ± 40.4 |
0.012 |
172.9 ± 37.2 |
181.7 ± 43.3 |
0.055 |
Triglyceride (mg/dL) |
110.9 ± 63.3 |
124.8 ± 89.1 |
0.189 |
119.9 ± 71 |
125.4 ± 97.7 |
0.85 |
Creatinine (mg/dL) |
1.18 ± 0.5 |
1.02 ± 0.29 |
0.018 |
1.07 ± 0.37 |
1.02 ± 0.30 |
0.23 |
Heart Rate |
84.87 ± 15.7 |
77.17 ± 20.9 |
0.001 |
80.37 ± 22 |
76.4 ± 18.6 |
0.324 |
SBP (mmHg) |
134.8 ± 38.4 |
129.7 ± 28.8 |
0.413 |
131.8 ± 30.9 |
129.3 ± 30.0 |
0.212 |
DBP (mmHg) |
83.16 ± 20.5 |
74.49 ± 15.7 |
0.376 |
81.3 ± 16.6 |
78.8 ± 16.4 |
0.16 |
Ejection Fraction (%) |
38.93 ± 10.1 |
43.32 ± 9.18 |
0.015 |
42.2 ± 9.31 |
43.1 ± 9.5 |
0.314 |
Table 1: Baseline characteristics of studied groups; Numerical variables are presented as mean ± SD (standard deviation), and categorical variables are summarized by raw numbers and percentages.
aVR-STD: aVR ST Devation; aVR STE: aVR ST Elevation; CAD: Coronary Artery Disease; CKMB: Creatine phosphokinase type MB; DBP: Diastolic Blood Pressure; HDL: High Density Lipoprotein Cholesterol; LDL: Low Density Lipoprotein Cholesterol; LV: Left Ventricle; SBP: Systolic Blood Pressure; STEMI: ST Elevation Myocardial Infarction; VS: Versus
In-hospital outcome: A comparison between the in-hospital outcomes of the included patients according to aVR-STE or aVR-STD in patients is given in Table 2. The primary endpoint of the study (in-hospital death) was not significantly different in patients with or without aVR-STE [four patients (8.2%) with aVR-STE and 13 (4.6%) patients without aVR-STE, RR: 1.790, 95% confidence interval: 0.608-5.264, P = 0.290]. The in-hospital mortality was almost the same in patients with or without aVR-STD (Table 2) and the incidence of death was not significantly different among different types of STEMI (involving the anterior, inferior or lateral wall) in patients with aVR-STE or aVR-STD (Table 2).
All of the Patients |
aVR-STE n=49 |
Without aVR-STE |
P value |
aVR-STD n=159 |
Without aVR-STD n=175 |
P value |
Death |
4 (8.2%) |
13 (4.6%) |
0.29 |
8 (5%) |
9 (5.1%) |
0.963 |
R-STEMI |
0 |
0 |
0.999 |
0 |
0 |
0.999 |
RI-CP |
9 (18.4%) |
50(17.5%) |
0.889 |
26 (16.4%) |
33 (18.9%) |
0.549 |
PE-HF |
7 (14.3%) |
12 (4.2%) |
0.012 |
13 (8.2%) |
6 (3.4%) |
0.061 |
Composite End Point |
19 (38.8%) |
70 (24.6%) |
0.038 |
43 (27%) |
46 (26.3%) |
0.876 |
Anterior wall STEMI (n=196) |
aVR-STE n=42 |
Without aVR-STE |
P value |
aVR-STD n=86 |
Without aVR-STD n=110 |
P value |
Death |
3 (7.1%) |
8 (5.2%) |
0.705 |
5 (5.8%) |
6 (5.5%) |
>0.999 |
R-STEMI |
0 |
0 |
>0.999 |
0 |
0 |
>0.999 |
RI-CP |
8 (19.0%) |
32(20.8%) |
0.805 |
16 (18.6%) |
24 (21.8%) |
0.58 |
PE-HF |
7 (16.7%) |
6 (3.9%) |
0.008 |
9 (10.5%) |
4 (3.6%) |
0.057 |
Composite End Point |
17(40.5%) |
43 (27.9%) |
0.118 |
27 (31.4%) |
33 (30.0%) |
0.833 |
Inferior wall STEMI (n=133) |
aVR-STE n=6 |
Without aVR-STE |
P value |
aVR-STD n=70 |
Without aVR-STD n=63 |
P value |
Death |
1 (1.7%) |
5 (3.5%) |
0.246 |
3 (4.3%) |
3 (4.8%) |
>0.999 |
R-STEMI |
0 |
0 |
>0.999 |
0 |
0 |
>0.999 |
RI-CP |
0 (0.0%) |
18(14.2%) |
>0.999 |
9 (12.9%) |
9 (14.3%) |
0.81 |
PE-HF |
0 (0.0%) |
5 (3.9%) |
>0.999 |
4 (5.7%) |
1 (1.6%) |
0.369 |
Composite End Point |
1(16.7%) |
26 (20.5%) |
>0.999 |
15 (21.4%) |
12 (19.0%) |
0.733 |
Lateral wall STEMI (n=35) |
aVR-STE n=3 |
Without aVR-STE |
P value |
aVR-STD n=26 |
Without aVR-STD n=9 |
P value |
Death |
1 (33.3%) |
0 (0.0%) |
0.086 |
1 (3.8%) |
0 (0.0%) |
>0.999 |
R-STEMI |
0 |
0 |
0.999 |
0 |
0 |
>0.999 |
RI-CP |
1 (33.3%) |
7 (21.9%) |
0.553 |
8 (30.8%) |
0 (0.0%) |
0.081 |
PE-HF |
0 (0.0%) |
4 (12.5%) |
>0.999 |
3 (11.5%) |
1 (11.1%) |
>0.999 |
Composite End Point |
2 (66.7%) |
10 (31.2%) |
0.266 |
11 (42.3%) |
1 (11.1%) |
0.121 |
Table 2: Results of in-hospital follow up in studied groups.
aVR-STD: aVR ST Deviation; aVR-STE: aVR ST Elevation; PE-HF: Pulmonary Edema or overt decompensated Heart Failure; RI-CP: Recurrent Ischemic Chest Pain; R-STEMI: Recurrent ST-Elevation Myocardial Infarction
In total, 89 (26.65%) patients had ≥ 1 event associated with in-hospital composite endpoints. The in-hospital composite endpoint occurred more in patients with aVR-STE (Table 2, RR: 1.579, 95% confidence interval: 1.051-2.370, P = 0.038), but it was not significantly different in patients with or without aVR-STD (Table 2). Multivariate analysis showed that, among the included confounding variables in the model, ST elevation in aVR was not an independent predictor of in-hospital composite endpoint (Table 3A). Neither aVR-STE nor aVR-STD had a relationship to in-hospital recurrent STEMI and recurrent chest pain, although PE-HF was seen more in patients with aVR-STE [seven patients (14.3%) with aVR-STE, versus 12 patients (4.2%) without aVR-STE, RR: 3.393, 95% confidence interval: 1.405-8.192, P = 0.012] and it trended more in patients with aVR-STD (Table 2, RR: 2.385, 95% confidence interval: 0.929-6.124, P=0.06). Multivariate analysis showed that ST elevation in aVR was an independent predictor of in-hospital PE-HF [Ex (B): 38.46, 95% confidence interval: 1.014-1000, P = 0.04 Table 3B].
Variable |
Ex (B) |
95% Confidence Interval for Ex(B) |
P value |
A: In-hospital cumulative endpoint |
|||
Family history of premature CAD |
5.556 |
1.587-14.286 |
<0.0001 |
Blood urea nitrogen |
1.054 |
1.018-1.090 |
0.003 |
Summed ST elevation |
1.054 |
1.009-1.101 |
0.019 |
Serum potassium |
2.017 |
0.918-4.432 |
0.081 |
B: In-hospital pulmonary edema and overt decompensated heart failure |
|||
Treatment with diuretics |
333.333 |
4.27-10000 |
0.009 |
Summed ST elevation |
1.167 |
1.036-1.315 |
0.011 |
Treatment with beta blockers |
0.008 |
0.0001-0.409 |
0.016 |
Aspartate transaminase |
1.010 |
1.001-1.018 |
0.027 |
ST elevation in lead aVR |
38.46 |
1.014-1000 |
0.049 |
Blood urea nitrogen |
1.025 |
1.000-1.050 |
0.050 |
Serum potassium level |
7.383 |
0.890-61.225 |
0.064 |
Ejection fraction |
0.867 |
0.744-1.010 |
0.066 |
Table 3: Results of the final step of binary logistic regression with backward Wald method for A: independent predictors of in-hospital cumulative endpoint. B: In-hospital pulmonary edema and overt decompensated heart failure.
CAD: Coronary Artery Disease.
Six-month follow up: During six-month follow up, mortality was not significantly different among patients with or without aVR-STE or ST deviation (Table 4). Composite endpoints trended more in patients with aVR-STE (Table 4, RR: 1.262, 95% confidence interval: 0.940-1.695, P = 0.149), and it was not significantly different among patients with or without aVR-STD (Table 4). Multivariate analysis using Cox regression analysis showed that aVR-STE was not an independent predictor of a six-month composite endpoint (Table 5A).
All of the Patients |
aVR-STE n=48 |
Without aVR-STE |
P value |
aVR-STD n=150 |
Without aVR-STD n=166 |
P value |
Death |
4 (8.3%) |
25 (9.3%) |
>0.999 |
14 (9.3%) |
15 (9%) |
0.927 |
R-STEMI |
0 (0%) |
2 (0.7%) |
>0.999 |
1 (0.7%) |
1 (0.6%) |
>0.999 |
RI-CP |
10 (20.8%) |
67(25.0%) |
0.536 |
34(22.7%) |
43(25.9%) |
0.503 |
PE-HF |
12 (25%) |
21 (7.8%) |
<0.001 |
21 (14%) |
12 (7.2%) |
0.049 |
Composite End Point |
26 (54.2%) |
115 (42.9%) |
0.149 |
70 (46.7%) |
71 (42.8%) |
0.487 |
Anterior wall STEMI |
aVR-STE n=41 |
without aVR-STE |
P value |
aVR-STD n=80 |
Without aVR-STD n=102 |
P value |
Death |
3 (7.3%) |
15 (10.6%) |
0.767 |
8 (10%) |
10 (9.8%) |
0.965 |
R-STEMI |
0 |
1 (0.7%) |
>0.999 |
0 |
1 (1%) |
>0.999 |
RI-CP |
9 (22%) |
38 (27%) |
0.52 |
18 (22.5%) |
29 (28.4%) |
0.364 |
PE-HF |
9 (22%) |
12 (8.5%) |
0.026 |
13 (16.2%) |
8 (7.8%) |
0.078 |
Composite End Point |
21 (51.2%) |
66 (46.8%) |
0.619 |
39 (48.8%) |
48 (47.1%) |
0.821 |
Inferior wall STEMI |
aVR-STE n=6 |
Without aVR-STE |
P value |
aVR-STD n=67 |
Without aVR-STD n=62 |
P value |
Death |
1 (16.7%) |
10 (8.1%) |
0.421 |
6 (9%) |
5 (8.1%) |
0.856 |
R-STEMI |
0 |
1 (0.8%) |
>0.999 |
1 (1.5%) |
0 |
>0.999 |
RI-CP |
0 |
28 (22.8%) |
0.339 |
14 (20.9%) |
14 (22.6%) |
0.817 |
PE-HF |
3 (50%) |
8 (6.5%) |
0.008 |
8 (11.9%) |
3 (4.8%) |
0.149 |
Composite End Point |
4 (66.7%) |
47 (38.2%) |
0.212 |
29 (43.3%) |
22 (35.5%) |
0.365 |
Lateral wall STEMI |
aVR-STE n=3 |
Without aVR-STE |
P value |
aVR-STD n=24 |
Without aVR-STD n=8 |
P value |
Death |
1 (33%) |
1 (3.4%) |
0.181 |
2 (8.3%) |
0 |
>0.999 |
R-STEMI |
1 (3.4%) |
0 |
>0.999 |
1 (4.2%) |
0 |
>0.999 |
RI-CP |
1 (33.3%) |
9 (31.0%) |
>0.999 |
9 (37.5%) |
1 (12.5%) |
0.38 |
PE-HF |
0 |
5 (17.2%) |
>0.999 |
3 (12.5%) |
2 (25%) |
0.578 |
Composite End Point |
2 (66.7%) |
16 (55.2%) |
>0.999 |
15 (62.5%) |
3 (37.5%) |
0.252 |
Table 4: Results of six-month follow up in studied groups with completed follow up.
aVR-STD: aVR ST Deviation, aVR-STE: aVR ST Elevation, PE-HF: Pulmonary Edema or overt decompensated Heart Failure, RI-CP: Recurrent Ischemic Chest pain, R-STEMI: Recurrent ST-Elevation Myocardial Infarction
Among the endpoints, PE-HF occurred more in patients with aVR-STE and aVR-STD (RR: 3.190, 95% confidence interval: 1.684-6.046, P < 0.0001 and RR: 1.937, 95% confidence interval: 0.987-3.800, P = 0.049 respectively, Table 4), but death, recurrent STEMI and recurrent chest pain were not significantly different (Table 4). Multivariate analysis showed that neither aVR-STE nor aVR-STD were independent predictors of PE-HF in six-month follow up (Table 5B).
Variable |
Ex (B) |
95% confidence interval for Ex (B) |
P value |
A: 6-month composite endpoints |
|||
Serum potassium |
1.494 |
0.977 – 2.286 |
0.064 |
Family history of premature CAD |
1.626 |
0.914 – 2.890 |
0.098 |
B: 6-month pulmonary edema and decompensated heart failure |
|||
Blood urea nitrogen |
1.057 |
1.030-1.086 |
<0.0001 |
Hyperlipidemia |
20.833 |
4.500- 10 |
<0.0001 |
Cigarette smoking |
4.367 |
1.275-14.925 |
P=0.019 |
Table 5: Final step of Cox regression using backward Wald method for independent predictors of A: 6-month composite endpoints (death, recurrent STEMI, recurrent chest pain, pulmonary edema and decompensated heart failure), B: 6-month pulmonary edema and decompensated heart failure in the follow up.
CAD: Coronary Artery Disease.
Kaplan meier curves for death, heart failure and composite end point according to aVR-STE or aVR-STD are given in Figure 1. The mean duration of hospital stay was 6.6 ± 2.47 days in patients with aVR-STE versus 5.92 ± 2.38 days in those without aVR-STE (P = 0.01), and 6.24 ± 2.40 days in patients with aVR-STD versus 5.83 ± 2.40 days in patients without aVR-STD (P = 0.124).
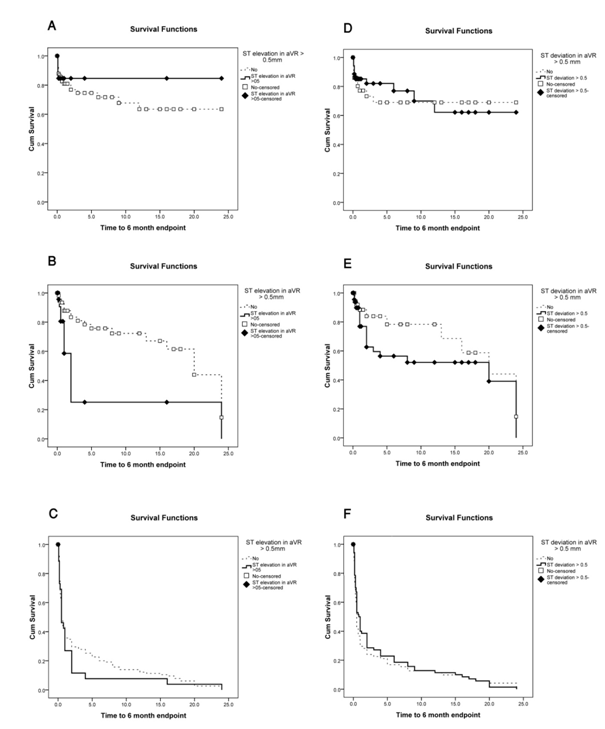
Figure 1: Kaplan-meier curves for 6 month follow up.
A: 6 month occurrence of death according to aVR ST elevation,
B: 6- month occurrence of pulmonary edema and decompensated heart failure according to aVR ST elevation,
C: 6-month occurrence of composite end point according to aVR ST elevation,
D: 6 month occurrence of death according to aVR ST deviation,
E: 6- month occurrence of pulmonary edema and decompensated heart failure according to aVR ST deviation,
F: 6-month occurrence of composite end point according to aVR ST deviation.
The incidence of aVR-STE in our study was 14.67%, which was concordant with prior work [14]. Some studies-but not all of them [22]-have shown that ST elevation in lead aVR has been an indicator of poorer prognosis and increased mortality in STEMI [1,4,12,14,20,21]. HERO-2 investigators studied 15,315 STEMI patients who received streptokinase and showed that ST elevation in aVR was associated with higher 30-day mortality regardless of the location of infarction [1,21]. However, in our study neither aVR-STE nor aVR-STD were predictors of in-hospital and six-month mortality (Tables 2 & 4). In the present study, aVR-STE was related to in-hospital composite adverse events in univariate analysis (P = 0.038, Table 2), whereas aVR-STD was not (P = 0.876, Table 2). Analysis using different types of STEMI showed that this pattern was seen mostly in anterior STEMI; however, our study was not sufficiently powerful to draw a definite comparison of the different types of STEMI. In our study, ST depression in lead aVR was seen more in inferior wall infarction. This may explain why, in our study, aVR-STD did not influence in-hospital composite adverse events (P = 0.876, Table 2). More studies are needed to confirm this.
Six-month follow up showed that neither aVR-STE nor aVR-STD were related to six-month composite endpoints (P = 0.149 and P = 0.487 respectively, Table 4). This finding was in agreement with the finding of Senaratne et al. [22], who concluded that aVR-STD was not related to the incidence of one-year adverse cardiac events [22].
Among in-hospital outcomes, neither aVR-STE nor aVR-STD was related to in-hospital recurrent ischemic chest pain and recurrent STEMI (Table 2). It seems that the major difference and relative superiority of the in-hospital composite endpoint in patients with aVR-STE is drawn from PE-HF. ST elevation in lead aVR was related to both in-hospital and six-month PE-HF (P < 0.05, Tables 2 & 4), and aVR-STD tended (P = 0.061, Table 2) to affect its in-hospital occurrence and was significantly related to six-month follow up (P = 0.049). These results were more prominent in anterior STEMI (Tables 2 & 4). There are some other studies indicating that in STEMI [14] and NSTEMI [2], aVR-STE has been associated with a worse Killip class at hospital admission and lower ejection fraction [14]. ST depression in lead aVR has been useful for predicting larger infarction and left ventricular dysfunction (lower ejection fraction) in patients with anterolateral STEMI [23]. However, this finding is not supported by some other studies [15]. The mean ejection fraction in our study was less in patients with aVR-STE (P = 0.015, Table 1), but it was not significantly different in patients with or without aVR-STD (P = 0.314, Table 1).
Patients with aVR-STE needed significantly more diuretic therapy (P = 0.018, Table 1), also those with aVR-STD tended to need more diuretic therapy, P = 0.105, Table 1) during in-hospital admission. This finding is in accordance with more in-hospital PE-HF in those with aVR-STE. A possible description of this finding in our study may be the greater involvement of the anterior wall in patients with aVR-STE and less involvement of the inferior wall (P = 0.0001, Table 1). In addition, there might be a possibility of greater extensive myocardial infarction in patients with aVR-STE or aVR-STD. More studies are needed to confirm this finding because our study was not powerful enough to determine the independent predictors of PE-HF. In the present study, the mean duration of hospital stay was more in patients with aVR-STE (P = 0.01). This finding is probably due to more in-hospital composite endpoints (P = 0.038, Table 2) and particularly heart failure in this group (P = 0.012, Table 2). The mean hospital stay also tended to be more in patients with aVR-STD; however, this was not statistically significant, which is in agreement with some other studies [22]. This finding is in concert with the finding that neither composite endpoints nor PE-HF were significantly related to aVR-STD.
In our study, patients with aVR-STE had higher heart rates (P = 0.001, Table 1). There are other studies which show that aVR-STE has been associated with a higher heart rate and lower systolic blood pressure [14]. This may be explained by a lower ejection fraction (Table 1), a higher prevalence of in-hospital heart failure (Table 2), and – possibly –a greater extent of infarction in these patients. As is shown in Table 1, the summed ST elevation and summed ST deviation were more in those patients with aVR-STE and aVR-STD. This finding is important, because the summed ST elevation and ST deviation are important predictors of the outcome and extent of myocardial infarction. To understand which one is the more powerful and the more important predictor of outcome requires more study.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.