Journal of
eISSN: 2373-6437


Caesarean section (C-section) is one of the most rear operations in rabbit. The present case study represents the efficacy of the surgical outcome and anesthetic protocol of C-section in rabbit. A 1.2 years old, local and non-descriptive breed of rabbit was presented to the SAQ Teaching Veterinary Hospital (SAQTVH), Chittagong Veterinary and Animal Sciences University (CVASU) with a history of one kit normally delivered and remains straining and treated with oxytocin. Problem on physical examine included abdominal distension and vaginal secretion. Based on the clinical examination, X-Ray and Ultrasonography report, it was decided to perform surgery. The surgery was aseptically control under general anesthesia combination with xylazine and ketamine with the dose rate 5mg/kg and 30mg/kg respectively. The rabbit became unconscious within 3 minutes of intramuscular injection. Laparotomy midline incision (1cm) was performed behind the umbilicus. Two dead fetuses were removed and proper apposition of abdomen was done. The rabbit was recovered fully after 50 minutes of injection. As a part of post operative treatment which was maintained with antibiotic, pain killer for 7 days. The wound healing was noticed after 10 days of operation. So, the case report suggests that rabbit C-section can be performed successfully with xylazine and ketamine anesthesia with recommended doses.
Keywords: rabbit, anaesthesia, caesarean section
Bunny fit to small mammals’ in the family leporidae of the order lagomorph, There are about fifty different species of bunny found in many parts of the world.1 Rabbits are popular pets which will be seen by the small animal practitioner. A C-section is often necessary for bunny when delivery per vagina is too difficult and when all other methods to deliver the kit naturally have failed, and therefore could endanger the life of the bunny or the life of her kit.2 The ideal goals in performing a cesarean section are survival of the rabbit and kit, and maintenance of the future reproductive efficiency of the rabbit. The rabbit which is close to parturition should be checked frequently for signs of dystocia. They include tenacious contractions, bloody discharge, straining with no obvious progress, or a green-brown discharge from the vagina.3 Dystocia revenues the incapability to exorcize fetus from the uterus even after assumption of full term and may be due to maternal or fetal causes.4 Dystocia was not collective in rabbits as normal delivery was accomplished within thirty minutes after the onset.5 Communal causes of dystocia in rabbits included obesity, oversized kids, narrow pelvic canal or uterine inertia.6 Female rabbits C-section in dorsal recumbency via a ventral midline incision was Following the initial skin incision, the subcuticular tissue is bluntly dissected to expose the linea alba.10 This paper reports the successful management of anesthesia in a Bunny doe with cesarean section.
On 22 June 2017, a 10 months-old, bearing weight 1.5kg local rabbit breed was brought to the Teaching Veterinary Hospital (TVH) of Chittagong Veterinary and Animal Sciences University (CVASU), Chittagong with complain, staining and vaginal discharge remain and treated with oxytocin and calcium before 12 hours. Clinical examinations, diagnostic techniques with x-ray noticed 2 fetus remain. The clinical parameters respiratory rate, rectal temperature, heart rate were normal ranges (Table 1).
Mental status |
Calm, Weakness |
General appearance |
Straining with an edematous vulva and bloody discharge from vagina |
MM color |
Pink |
Cardiovascular |
HR 135 bpm, CRT<2 sec |
Body temperature |
100.8˚ F |
Table 1 Clinical parameters of a rabbit
Restraining anaesthesia with and close monitoring
General anesthesia was obtained by injecting xylazine hydrochloride (Inj. Xylazine® - 5 mg/kg body weight) intramuscularly followed by ketamine hydrochloride (Inj. G-ketamine®-30mg/kg body weight) 1minutes interval respectively and regularly monitoring heart rate and eye reflexes (Figure 1).
Surgical procedure
The Rabbit was anaesthetized by intramuscular injection of 0.37 mL (@5mg/kg body weight xylazine (xylaxin®, 20 mg/mL, India Immunologicals Ltd., Hyderabad, India), 0.9 mL ketamine (G-ketamine®, 30 mg/mL, Gonoshasthaya Pharmaceuticals Ltd., Mirzanagar Dhaka, Bangladesh). The pet became unconscious within two minutes as soon as the pet was anesthetized. The temperature, heart rate and respiration are monitored throughout the procedure. The Naval area of about 7 cm x 8 cm was shaved and cleaned the surgical area was scrubbed with 7.5% povidone iodine solution. This was repeated for 3 times. Later, when the pet was shifted to the operation theatre the area was again sterilized with 7.5% surgical povidone iodine solution and surgical spirit alternatively twice. The surgery was aseptically controlled under general anesthesia. Laparotomic mid line incision 1 cm behind the umbilicus was performed. At first 3 cm long incision was made on skin. The bleeding was checked by applying gauge pressure and artery forceps. Make incision in mid of uterus and and make suture in proper ways catgut (1-0). (Trugut®, Suture India Pvt. Ltd., Bangalore, India) The skin was then closed with cross-mattress suture pattern using silk. The sutured wound was covered with the benzoin seal (Figure 2).
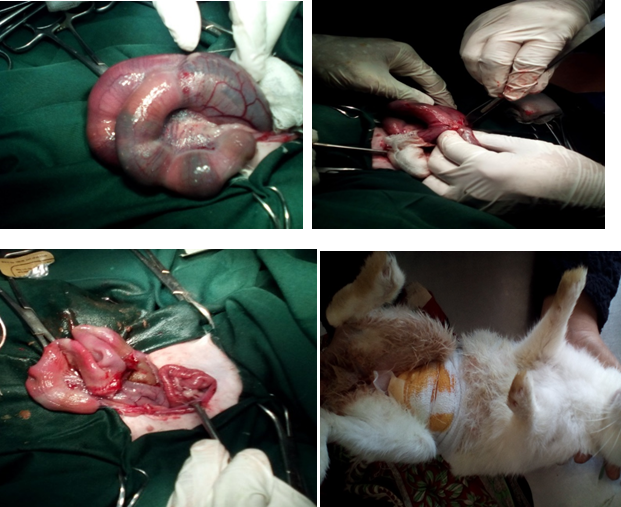
Figure 2 Surgical procedure of caesarian section in rabbit. (A) Gravid uterus. (B) Incision in the uterus. (C) Suturing of uterus. (D) Condition after surgery.
Postoperative treatment and care
The owner was advised to keep the surgical site clean and apply Povidone iodine ointment twice daily and Antibiotic Ceftriaxone @60 mg /Kg bodyweight (Inj. Ceftron 250mg®, Square Pharmaceuticals, Bangladesh) was administered intramuscularly daily for7 days. Antihistaminic Chlorpheneramine maleate @1mg/Kg body weight (Inj. Renacin®, Renata Ltd, Bangladesh) was administered intramuscularly daily for 7days. Analgesic Meloxicam @0.2 mg/Kg body weight (Inj.Melvet®, Acme Laboratories Ltd., Bangladesh) was administered subcutaneously daily for 5 days for pain management. The patient was monitored and observed for 14 days under a Veterinarian. No complication was noted and the rabbit recovered uneventfully. On the 14th day, the suture was removed and it was noticed that the surgical site was healed completely.
Female rabbit reproductive tract is unique as it lacks a uterine body and each uterine horns has its own cervix which opens directly into the vagina.5 Dystocia is not common in rabbits as normal delivery is typically completed within 30 min after onset.5,8,9 Obesity, oversized kits, a narrow pelvic canal or uterine inertia are included as the common causes of dystocia in rabbits5,6,8 but in my case study 1 kit normally delivery and 2 kits remain in uterus. Oxytocin promotes the influx of calcium into the myometrial cells, increasing the frequency and strength of uterine contractions. In rabbits, doses of 1 to 3 units of oxytocin can be administered intramuscularly to assist in uterine contraction. Calcium gluconate may also be used as an uterotonic agent in combination with oxytocin.2 But in my study case no response of calcium gluconate and oxytocin it may be due to dead of fetus.In my study 30mg/kg/bw/Im ketamineand xylazine 5mg/kg/bw/im as postoperative meloxicam 0.2mg/kg/bw/im.Analgesia, Non-steroidal antiinflammatory which support my study15-20mg/kg/iv,20-50mg/kg/im and xylazine 1-5mg/kg/bw/im as meloxicam 0.2mg/kg/bw im5 rabbit anaesthesia with ketamine and xylazine is safe for caesarian section with recomanded does.10
Rabbit are one of the small pet mammals for the recreation of high class people. This paper will be helpful for field veterinarian and owner to know how they can care their pet rabbit. C-section can be considered as 100% safe for dystocia rabbit rather than forceful manual traction.
The author is thankful for Professor Dr. Bhajan Chandra das, medicine and surgery department.
Authors declare there is no conflict of interest in publishing the article.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.