Journal of
eISSN: 2572-8466


Research Article Volume 5 Issue 4
Department of Chemistry, University of Nairobi, Kenya
Correspondence: Kamau JM, Department of Chemistry, School of Biological Sciences, University of Nairobi, 30197-00100 NAIROBI, Kenya, Tel 254724305124
Received: May 10, 2018 | Published: July 27, 2018
Citation: Kamau JM, Mbui DN, Mwaniki JM, et al. Utilization of rumen fluid in production of bio–energy from market waste using microbial fuel cells technology. J Appl Biotechnol Bioeng. 2018;5(4):227-231. DOI: 10.15406/jabb.2018.05.00142
Environmental Protection Agency classifies slaughter house waste as one of the most toxic environmental pollutants due to high pathogen content. Composting and anaerobic digestion are among the most common methods used for its disposal. In this study, utilization of rumen fluid as bio– catalyst in microbial fuel cells is investigated. Different market wastes were converted to electricity by loading them in anodic anaerobic chamber and then adding rumen fluid from Dagoretti slaughterhouse. 0.584V was obtained on day 19 from avocado fruit waste while the maximum voltage for tomato waste was 0.701V on day 20. Water melon and fruits mixture produced the least voltage. The maximum power from the tested substrate was obtained from tomato wastes. The power and current density were in the range of 1.825 to 60.041mW/m2 and 6.762 and 99.174mA/m2 respectively for tomato wastes. A maximum voltage of 0.584V was obtained from tomato wastes when 500ml rumen fluid was used while 0.248Vwas obtained for avocado fruit waste with the same amount of rumen fluid. Electrode surface area of 0.006666m2 gave the highest voltage and power amongst 0.00399m2 and 0.01331m2. When the influence of external resistors was investigated, power, voltage and current obtained across a 45kΩ were 0.385V, 0.038Ma and 0.01463mW on day 7 respectively for tomato wastes.
Keywords: rumen fluid, voltage, current, resistance, microbes, market wastes
Slaughter house and meat processing industries discharge the most harmful wastes to water bodies and the environment at large.1 Discharging of these wastes without treatment greatly affect aquatic life as well as polluting drinking water.2 Slaughter house wastes are considered to have bacterial and viral pathogens which may infect both humans and animals with water borne diseases.3 Researchers have investigated different methods used in abattoir waste disposal. Effectiveness and efficiency of the method used is considered with regards to pathogen inactivation.3 Commonly used methods include alkaline hydrolysis, incineration, and composting, anaerobic digestion. Among these methods, only alkaline hydrolysis completely deactivates the microbes.3 In the alkaline hydrolysis method, biological substances are hydrolyzed using sodium or potassium hydroxide. This new technology employs temperature of 100°C and pressure of 103 kpa for 3h to destroy pathogens.4,5 In anaerobic digestion, organic wastes are degraded in an oxygen free environment. This produces biogas and agricultural sludge.6,7 Dagoretti slaughterhouse produces thousands of liters of rumen fluid daily. The fluid consists of methanogenic bacteria which could be used for biogas production as well as bio–catalyst in microbial fuel cells.8 Currently, the fluid from Dagoretti and Kiamaiko slaughter houses are dumped into the drainage system and washed away to Nairobi River. This has pollution effects considering that the water is used for domestic purposes. Both market and slaughter houses wastes have been associated with water borne diseases. This study proposes an alternative for slaughter houses wastes disposal like composting, incineration.3
Microbial fuel cells setups were fabricated using readily available materials as described by Kamau et al.9 The salt bridge was made up of 3% agarose in sodium chloride. Graphite rods from used dry cells were used as electrodes. The fruits used in this study were spoilt and collected from Kangemi and Wakulima market wastes bin.
Fruit wastes with rumen fluid
To investigate the potential of rumen fluid in digestion of fruit wastes for electricity generation, 500g of water melon, avocado, banana, tomato and mango was chopped and blended using a kitchen blender and added to the anodic chamber of H–shaped microbial fuel cells (MFC). Rumen fluid (250ml) from Dagoretti slaughter house was added and mixed. The anodic chamber was sealed tightly after attaching the electrode attached to the copper wire. About 750ml distilled water was added to the cathodic chamber with the electrode attached to the copper wire. A mixture of about 100g of water melon, avocado, banana, tomato and mango was added to the anodic chamber before introducing 250ml rumen fluid. In others set ups, a mixture of mango and avocado was added 250ml, 350ml and 500ml rumen fluid. The set–ups were sealed and linked to the cathodic chamber with a salt bridge. Current and voltage were recorded daily using a digital voltmeter.
Microbial fuel cells parameter optimization
Studies were carried out to optimize MFC operation conditions. These studies involved use of avocados and varying electrode surface area, varying external resistance as well as varying rumen fluid inoculums concentrations in tomatoes and avocado wastes. The procedures are described.
Electrode surface area
Avocado (500g) was chopped and blended before adding to the anodic chamber. Rumen fluid (500ml) was added to the same chamber with the avocado and thoroughly mixed. The electrodes were assembled as shown in Figure 1. Linking the anodic chamber to the cathodic chamber was done using a salt bridge with 1000ml distilled water being added to the cathodic chamber.
External resistance
To investigate the effect of external resistance, a H–shaped double chamber was fabricated. Anodic chamber was loaded with 500g avocado and 250ml rumen fluid. In another set, 500g of blended tomatoes was used in place of avocado. 1kΩ, 2kΩ, and 45 kΩ resistors were attached to both terminals of the copper wire and current and voltage recorded.
Rumen
To study the effect of microbe’s concentrations, the anodic chamber was fed with 500g avocado and tomato each, minced and blended and 250ml, 300ml and 500ml rumen fluid added. The set–up were fabricated as described above and current and voltage monitored and recorded daily.
Circuit assembly
The assembly of the H–shaped MFC was done as described by Kamau et al.10 A digital voltmeter was attached to the copper wires from the cathodic and anodic chambers with voltage and current being monitored on daily basis.
Statistical analysis
The reported data was collected in triplicates. Only mean is reported with standard deviation ranging between 0.028 and 2.569.
The voltage and power obtained when tomato and avocado fruits wastes were used in MFC for 20 days are in appendix Table 1 while the plots are shown in Figure 2 & Figure 3 respectively. Highest voltage was obtained from 500ml rumen fluid in tomato fruits wastes for the entire study period. The voltage increased linearly for the first six days due to high digestion rate from high microbe concentration in the 500ml rumen fluid compared to the two lower concentrations. The lowest power was obtained from avocado incubator with 250ml rumen fluid.
Days |
Tomato |
Avocado |
||||
250ml |
300ml |
500ml |
250ml |
300ml |
500ml |
|
1 |
0.046 |
0.274 |
0.174 |
0.135 |
0.15 |
0.169 |
2 |
0.008 |
0.23 |
0.404 |
0.122 |
0.059 |
0.127 |
3 |
0.069 |
0.145 |
0.424 |
0.101 |
0.069 |
0.072 |
4 |
0.092 |
0.248 |
0.477 |
0.105 |
0.0007 |
0.08 |
5 |
0.124 |
0.251 |
0.546 |
0.12 |
0.016 |
0.076 |
6 |
0.16 |
0.195 |
0.0552 |
0.123 |
0.024 |
0.063 |
7 |
0.195 |
0.202 |
0.554 |
0.152 |
0.03 |
0.048 |
8 |
0.217 |
0.161 |
0.556 |
182 |
0.028 |
0.03 |
9 |
0.223 |
0.121 |
0.559 |
0.196 |
0.025 |
0.076 |
10 |
0.228 |
0.129 |
0.558 |
0.21 |
0.017 |
0.115 |
11 |
0.246 |
0.132 |
0.568 |
0.234 |
0.019 |
0.119 |
12 |
0.253 |
0.047 |
0.545 |
0.212 |
0.026 |
0.195 |
13 |
0.282 |
0.053 |
0.538 |
0.217 |
0.077 |
0.231 |
14 |
0.33 |
0.099 |
0.538 |
0.215 |
0.002 |
0.248 |
15 |
0.344 |
0.138 |
0.334 |
0.223 |
0.008 |
0.237 |
16 |
0.325 |
0.12 |
0.381 |
0.219 |
0.01 |
0.019 |
17 |
0.293 |
0.118 |
0.456 |
0.225 |
0.007 |
0.202 |
18 |
0.301 |
0.115 |
0.497 |
0.241 |
0.022 |
0.219 |
19 |
0.275 |
0.09 |
0.501 |
0.247 |
0.068 |
0.241 |
20 |
0.242 |
0.084 |
0.49 |
0.248 |
0.128 |
0.212 |
21 |
0.151 |
0.058 |
0.51 |
0.248 |
213 |
0.241 |
Table 1 Voltage for different rumen fluid concentration
Figure 4 shows a plot of daily voltage obtained when rumen fluid concentrations was varied for the fruits mixture. It is evident that 350ml of rumen fluid gave the highest voltage. This may be attributed to almost enough nutrients for the microbes for the entire study period. 500ml rumen fluid gave the highest voltage after the first 24hours. This is explained by the fact that microbes are competing for food translating to high rate electron production. As for the 250ml rumen fluid, the rate of voltage generation is constant for the entire study period. This is explained by almost enough substrate for the microbes with little competition for the available food. The current produced by the degradation of the fruits mixture substrate was highest when inoculated with 250ml rumen fluid set. As earlier indicated, this is explained by constant production of electrons from tomato and avocado 1:1 mixture. This translates to highest power (calculated using equation 3.1) production since power is a multiple of current and voltage as shown in Figure 5.
(2)
On varying the anodic electrode surface area, the voltage obtained is shown in appendix Table 2. The maximum voltage of 0.449V was obtained from avocado fruit wastes on day 15. Voltage, power and current from the three electrode surface areas studied are shown in Figure 6. The highest current was obtained from 0.01331m2 electrode surface area at 0.209mA on day 16 while the lowest was on day 1. This is explained by the fact that all the electrons generated from the degradation on of the fruit waste are able to secure adsorption site on the electrode surface for the bigger surface area. The current density indicates the number of electrons produced per unit surface area of electrode. The calculation of current density was done using equation 1.
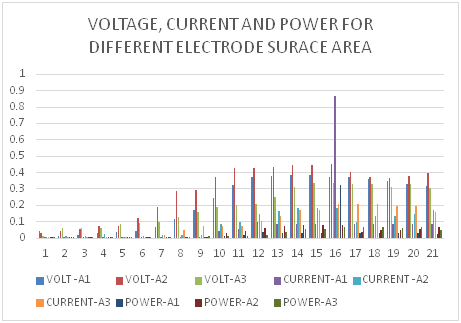
Figure 6 Voltage, current and power for A1-0.00399m2, A2-0.00666m2 and A3-0.01331m2 electrode surface area.
(1)
Where I is current and is the electrode surface area.
Figure 7 shows the current density for the different electrode surface areas. The power density obtained using equation 2 when electrodes of different surface area were used is shown in Figure 8. It is evident from the Figure that 0.00666m2 electrode surfaces gave the highest power density.

Figure 7 Current density plots for A1-0.00399m2, A2-0.00666m2 and A3-0.01331m2 electrode surface area.
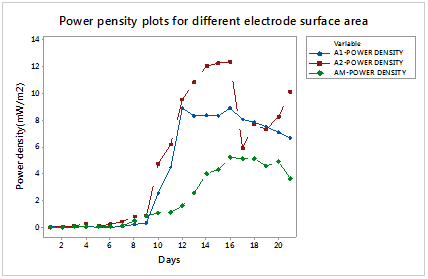
Figure 8 Power density plots for A1-0.00399m2, A2-0.00666m2 and A3-0.01331m2 electrode surface area.
(2)
On investigating the effect of external resistance on voltage production from the MFC, the voltage obtained across different resistors and open circuit voltage (OCV) is shown in appendix on Table 3 and a plot of the data is shown in Figure 9. OCV is the highest since only internal resistance is to be overcome. This internal resistance is from cathode, anode and electrolyte material. Previous studies by Menicucci et al.11using 6 to 0.125 KΩ revealed that, the cell voltage decreases when external resistance decreases. This is explained by the limitations imposed on the electrode reaction kinetics, on mass transfer, and on charge– transfer processes at the current– limiting electrode. In another study, Ghangrekar et al.,12 observed that cell voltage increased with an increase in external resistance from 0 to 4,000 Ω; the maximum voltage of 358 mV was observed at an external resistance of 4,000 Ω. Later on, Rismani–Yazdi et al.,13 2011 obtained similar cathode potentials at different external resistances. However, anode potential varied under different external employed. MFCs with lower external resistances resulted in higher anode potentials. This was also observed in the study of Song et al.,14 carried out using a sediment microbial fuel cell.
Days |
A1-0.00399m2 |
A2-0.00666m2 |
A3-0.01331m2 |
|||
Volt |
Current |
Volt |
Current |
Volt |
Current |
|
1 |
0.043 |
0.006 |
0.029 |
0.004 |
0.01 |
0.002 |
2 |
0.011 |
0.002 |
0.041 |
0.014 |
0.059 |
0.007 |
3 |
0.019 |
0.002 |
0.054 |
0.014 |
0.059 |
0.008 |
4 |
0.03 |
0.006 |
0.074 |
0.025 |
0.061 |
0.009 |
5 |
0.035 |
0.001 |
0.074 |
0.008 |
0.084 |
0.006 |
6 |
0.042 |
0.002 |
0.122 |
0.012 |
0.09 |
0.007 |
7 |
0.069 |
0.003 |
0.187 |
0.016 |
0.098 |
0.017 |
8 |
0.118 |
0.007 |
0.287 |
0.019 |
0.129 |
0.051 |
9 |
0.173 |
0.008 |
0.291 |
0.02 |
0.161 |
0.072 |
10 |
0.243 |
0.042 |
0.374 |
0.085 |
0.191 |
0.073 |
11 |
0.321 |
0.056 |
0.428 |
0.096 |
0.201 |
0.074 |
12 |
0.371 |
0.096 |
0.427 |
0.149 |
0.206 |
0.103 |
13 |
0.381 |
0.087 |
0.431 |
0.167 |
0.252 |
0.135 |
14 |
0.385 |
0.087 |
0.443 |
0.181 |
0.31 |
0.171 |
15 |
0.386 |
0.086 |
0.449 |
0.182 |
0.335 |
0.172 |
16 |
0.371 |
0.087 |
0.45 |
0.183 |
0.334 |
0.209 |
17 |
0.37 |
0.087 |
0.402 |
0.098 |
0.329 |
0.207 |
18 |
0.36 |
0.087 |
0.373 |
0.137 |
0.331 |
0.207 |
19 |
0.349 |
0.086 |
0.366 |
0.133 |
0.311 |
0.196 |
20 |
0.33 |
0.086 |
0.38 |
0.145 |
0.329 |
0.198 |
21 |
0.316 |
0.084 |
0.397 |
0.17 |
0.308 |
0.158 |
Table 2 Voltage and current for different surface area
Days |
Tomato |
Ovacado |
||||||
1KΩ |
2KΩ |
45KΩ |
OCV |
1KΩ |
2KΩ |
45KΩ |
OCV |
|
1 |
0.002 |
0.01 |
0.024 |
0.174 |
0.021 |
0.043 |
0.097 |
0.169 |
2 |
0.007 |
0.001 |
0.003 |
0.404 |
0.017 |
0.041 |
0.037 |
0.127 |
3 |
0.023 |
0.026 |
0.019 |
0.424 |
0.021 |
0.032 |
0.029 |
0.072 |
4 |
0.079 |
0.096 |
0.024 |
0.477 |
0.026 |
0.013 |
0.03 |
0.08 |
5 |
0.04 |
0.057 |
0.175 |
0.546 |
0.001 |
0.004 |
0.064 |
0.076 |
6 |
0.041 |
0.06 |
0.192 |
0.552 |
0.003 |
0.002 |
0.031 |
0.063 |
7 |
0.044 |
0.079 |
0.385 |
0.554 |
0.001 |
0.003 |
0.034 |
0.048 |
8 |
0.082 |
0.074 |
0.147 |
0.556 |
0.001 |
0.003 |
0.037 |
0.03 |
9 |
0.041 |
0.054 |
0.167 |
0.559 |
0.003 |
0.002 |
0.014 |
0.076 |
10 |
0.051 |
0.084 |
0.264 |
0.558 |
0.004 |
0.005 |
0.02 |
0.115 |
11 |
0.063 |
0.098 |
0.278 |
0.568 |
0.006 |
0.01 |
0.023 |
0.119 |
12 |
0.026 |
0.045 |
0.299 |
0.545 |
0.007 |
0.01 |
0.131 |
0.195 |
13 |
0.028 |
0.057 |
0.211 |
0.538 |
0.005 |
0.01 |
0.151 |
0.231 |
14 |
0.024 |
0.057 |
0.319 |
0.538 |
0.003 |
0.006 |
0.029 |
0.248 |
15 |
0.047 |
0.077 |
0.191 |
0.334 |
0.031 |
0.034 |
0.073 |
0.237 |
16 |
0.044 |
0.064 |
0.145 |
0.381 |
0.016 |
0.015 |
0.133 |
0.019 |
17 |
0.036 |
0.063 |
0.171 |
0.456 |
0.019 |
0.017 |
0.184 |
0.202 |
18 |
0.038 |
0.077 |
0.163 |
0.497 |
0.015 |
0.016 |
0.125 |
0.219 |
19 |
0.036 |
0.048 |
0.14 |
0.501 |
0.005 |
0.017 |
0.035 |
0.241 |
20 |
0.059 |
0.08 |
0.161 |
0.49 |
0.006 |
0.013 |
0.052 |
0.212 |
21 |
0.051 |
0.051 |
0.165 |
0.51 |
0.005 |
0.007 |
0.086 |
0.241 |
Table 3 Voltage across different resistors and OVC for tomato and avocado fruit wastes
Electrode surface area, external resistance and microbial inoculum size added to the fruit wastes influences the amount of potential and power derived from the MFC using rumen fluid. The rate at which microbes breakdown fruit waste is proportional to current and voltage obtained from the fruit wastes.
None.
The author declares there is no conflict of interest.

©2018 Kamau, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.