Journal of
eISSN: 2572-8466


Research Article Volume 5 Issue 3
Department of Microbiology, University of Delhi, India
Correspondence: Kavita Vasdev, Department of Microbiology, Gargi College, University of Delhi, Siri Fort Road, New Delhi-110049, India, Tel 91-11-26494544
Received: February 27, 2018 | Published: May 24, 2018
Citation: Dewasthale S, Mani I, Vasdev K. Microbial biofilm: current challenges in health care industry.J Appl Biotechnol Bioeng. 2018;5(3):160-164. DOI: 10.15406/jabb.2018.05.00132
Biofilm formation has become a significant problem in health industry and much research has been done for deeper understanding of the processes involved. Biofilms may form on living or non-living surfaces and can be prevalent in natural, industrial and hospital settings. The biofilm growth cycle includes bacterial adhesion at all levels, starting with the initial physical attraction of bacteria to a substrate, and ending with the eventual liberation of cell clusters from the biofilm matrix. Cells may also communicate via quorum sensing, which may in turn affect biofilm processes such as detachment. Terribly, the physiological nature of biofilm forming microbial cell is different than planktonic cell of the same organism, which, by contrast, are single-cell that may swim or float in a liquid medium. Biofilms have great importance for public health because of their role in certain infectious diseases and importance in a variety of medical device-related infections. Therefore, there is a need to develop novel, effective and specific antimicrobial substances, which can be utilized to diminish the biofilm associated pathogenicity in hospital and other public spaces. This article provides an overview of understanding of the biofilm formation and the role of genetic and environmental factors in the development of biofilm.
Keywords: biofilms, infections, microorganisms, quorum sensing, antimicrobial
Biofilms are communities of microorganisms that are attached to a surface and play a significant role in the persistence of bacterial infections. Biofilms have varied morphologies, depending on the constituent bacteria as well as the conditions under which that biofilm was formed.1 Bacterial biofilms are involved in a multitude of serious chronic infections. Joo & Otto2 demonstrated the in vitro modeling of biofilm infection that directed to the identification of microbial determinants leading to biofilm development. Biofilms are formed by a spectrum of microorganisms, including pathogens, and provide a means for these organisms to protect themselves against antimicrobial agents. Several mechanisms have been proposed to explain this phenomenon of resistance within biofilms.3 Biofilms formations are one of a major global challenge to control infection and healthcare-associated infections owing to their inherent tolerance and ‘resistance’ to antimicrobial treatments. They have been shown to develop on medical device surfaces, and dispersal of single and clustered cells indicates a major risk of microbial dissemination within the host and increased risk of infection (Figure 1).4
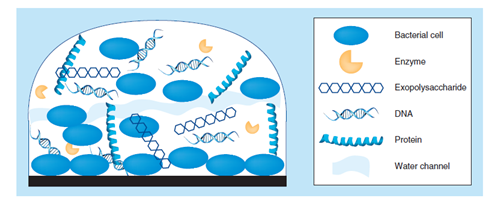
Figure 1 Biofilm structure of the extracellular polymeric substance (EPS) matrix of biofilms is composed of one or more of extracellular polysaccharides, DNA and proteins.5
The biofilms are composed of extracellular polymeric substance (EPS).5 The extracellular polymeric substance (EPS) matrix consists of exopolysaccharides, proteins and nucleic acids.5,6 A large proportion of the EPS is more or less strongly hydrated, though, hydrophobic EPS also occur; one example is cellulose7 that is produced by a range of microorganisms. This matrix encases the cells within it and facilitates communication among them through biochemical signals as well as gene exchange; the EPS matrix also traps extracellular enzymes and keeps them in close proximity of the cells. Some biofilms have been found to contain water channels that help distribute nutrients and signalling molecules.8 In addition, it was observed that extracellular DNA (eDNA) is a main structural component of many different microbial biofilms. Enzymatic degradation of extracellular DNA can weaken the biofilm structure and release microbial cells from the surface.9
Types of microorganisms involved
Generally, biofilms are formed by bacteria which colonize plants, e.g. Pseudomonas fluorescens and Pseudomonas putida, related pseudomonas spp., that are usual plant-associated bacteria found on roots, and leaves, in the soil, and the majority of their natural isolates form biofilms. Several nitrogen-fixing symbionts of legumes such as Rhizobium leguminosarum and Sinorhizobium meliloti form biofilms on legume roots and other inert surfaces. Along with bacteria, biofilms are also generated by archaeal.10
Many different bacteria form biofilms, including gram-positive (e.g. Bacillus spp, Listeria monocytogenes, Staphylococcus spp., and lactic acid bacteria, including Lactobacillus plantarum and Lactococcus lactis) and gram-negative species (e.g. Escherichia coli, or Pseudomonas aeruginosa). Cyanobacteria also form biofilms in aquatic environments.11
Establishment of a biofilm infection
Biofilm formation is commonly considered to occur in three main stages (Figure 2):
Biofilms develop via initial attachment, which depends on transport of the bacteria to a surface, which is passive in the case of non-motile bacteria such as staphylococci (yellow), and active in the case of motile bacteria such as P. aeruginosa (red).2 The interactions of motility appendages (flagella) with fluid and surfaces promote motility, attachment and dispersal of bacteria on surfaces prior to biofilm formation.12
Attachment
Attachment itself is governed by specific protein-protein interactions of bacterial surface with human matrix proteins. Attachment to an abiotic surface such as a catheter depends on bacterial surface hydrophobicity, but this mechanism is believed to have minor importance in vivo. Subsequent steps do not differ in principle between motile and non-motile bacteria. They involve proliferation, embedding in an extracellular matrix, and maturation. The latter depends on cell-cell disruptive factors, recently identified to be primarily surfactants. Strong production of surfactants, which are controlled by quorum sensing (QS), leads to biofilm detachment (dispersal). In the case of motile bacteria, up-regulation of motility, starting in the center of biofilm “mushroom caps” assists dispersal.13
Attachment to an abiotic surface such as a catheter depends on bacterial surface hydrophobicity, but this mechanism is believed to have minor importance in vivo. Extracellular DNAs (eDNAs) has an important role in biofilm formation (Figure 3). It is found to be critical for biofilm attachment.14
Visualization of labeled strands by using an anti-dsDNA monoclonal antibody conjugated to an immunogold particle (asterisks; scale bar = 250 nm).15
Matrix formation
After attachment to tissue or matrix-covered devices is accomplished, infectious bacterial biofilms grow by proliferation and production of an extracellular matrix. The function of the matrix is to provide adhesion between bacterial cells, thereby enabling the formation of a multilayered biofilm.
In vitro evidence indicates that the biofilm matrix consists of a multitude of components of different chemical nature, including exopolysaccharides, proteins, and other polymers. These components may also facilitate the formation of bacterial agglomerations that do not necessarily constitute a biofilm, and provide protection from antibiotics and host defenses independently of biofilm formation.16,17
Teichoic acids are characteristic major components of the cell surface in Gram-positive bacteria. Teichoic acids are negatively charged and have been shown to contribute to biofilm formation in staphylococci. Most likely, they interact with other surface polymers and function as a scaffold for protein attachment.18,19
Dispersal
Dispersal of cells from the biofilm colony is an essential stage of the biofilm life cycle. Dispersal enables biofilms to spread and colonize new surfaces. Enzymes that degrade the biofilm extracellular matrix, such as dispersin B and deoxyribonuclease, may play a role in biofilm dispersal. Biofilm matrix degrading enzymes may be useful as anti-biofilm agents. Recent evidence has shown that a fatty acid messenger, cis-2-decenoic acid, is capable of inducing dispersion and inhibiting growth of biofilm colonies. Nitric oxide has also been shown to trigger the dispersal of biofilms of several bacteria species at sub-toxic concentrations. Nitric oxide has the potential for the treatment of patients that suffer from chronic infections caused by biofilms.2
Quorum sensing
Biofilm development and quorum sensing (QS) are closely interconnected processes. Biofilm formation is a cooperative group behaviour that involves bacterial populations living embedded in a self-produced extracellular matrix. QS is a cell-cell communication mechanism that synchronizes gene expression in response to population cell density. Intuitively, it would appear that QS might coordinate the switch to a biofilm lifestyle when the population density reaches a threshold level. However, compelling evidence obtained in different bacterial species coincides in that activation of QS occurs in the formed biofilm and activates the maturation and disassembly of the biofilm in a coordinate manner.
In staphylococci (Figure 4), QS is established by the accessory gene regulator (Agr) system, which produces a secreted, post-translationally modified peptide that interacts with a two component system in an auto feedback loop, ultimately resulting in a considerable shift in gene expression patterns during early stationary growth phase. QS systems contribute to maturation and dispersal of biofilms. Accordingly, biofilms of an Agr QS wild-type strain contain channels between cellular agglomerations. Active expression of the QS system leads to dispersal. During prolonged chronic infection, the QS system in biofilms cells may be irreversibly inactivated by mutation, leading to excessive growth of compact biofilms, which likely have lost the capacity to disperse and disseminate. The phenotype of a surfactant mutant in which all psm genes controlled by Agr have been inactivated, has the same phenotype as the agr QS mutant underlining the importance of surfactants in QS-mediated control of biofilm maturation and detachment.2
QS systems (such as the staphylococcal Agr shown here) contribute to maturation and dispersal of biofilms. Accordingly, biofilms of an Agr QS wild-type strain, as shown by CLSM in the middle, contain channels between cellular agglomerations. Active expression of the QS system (as shown on the top right in green, using an agr promoter gfp fusion construct) leads to dispersal. During prolonged chronic infection, the QS system in biofilms cells may be irreversibly inactivated by mutation, leading to excessive growth of compact biofilms, which likely have lost the capacity to disperse and disseminate. The phenotype of a surfactant mutant, in which all psm genes controlled by Agr have been inactivated (bottom right), has the same phenotype as the agr QS mutant (bottom left), underlining the importance of surfactants in QS-mediated control of biofilm maturation and detachment.2
Significance of biofilms in health care
Biofilms are of great importance in infection control and healthcare-associated infections owing to their inherent tolerance and 'resistance' to antimicrobial therapies. Biofilms have been shown to develop on medical device surfaces, and dispersal of single and clustered cells implies a significant risk of microbial dissemination within the host and increased risk of infection Healthcare-associated infections (HCAIs) can occur in care homes, hospitals or in a patient’s own home.
The micro-organisms most frequently associated with HCAIs include Gram-positive bacteria, such as Staphylococcus aureus, Staphylococcus epidermidis and Enterococcus faecalis; Gram-negative bacteria, including Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis and Pseudomonas aeruginosa, and yeasts, particularly Candida species (Table 1).20,21
HCAI |
Micro-organism |
Medical device related |
|
CAUTI |
Coagulase negative stephylococci (CNS), C. albicans, A. |
Centralline associated septicaemia |
CNS, C. albicans, K. pneumoniae, P. aeruginosa, S. aerus, S. epidermidis |
VAP |
Candida, K. pneumoniae, P. aeruginosa, S. aerus, S. epidermidis |
Surgical‒site infection |
|
Surgical wound, prosthesis related infection |
Candida, E.coli, stephylococcus spp., MRSA, P. aeruginosa, S. aerus, S. epidermidis |
Table 1 Overview of most commonly isolated microorganisms isolated in biofilm related Healthcare-associated infections (HCAI)4
CAUTI, catheter-associated urinary tract infection; VAP, ventilator-associated pneumonia; MRSA, methicillin-resistant staphylococcus aureus
It is the growth of these microorganisms within biofilms that has posed a challenge in treating HCAIs, owing to the association of biofilms with increased resistance to antimicrobial therapies.22
The growth of microorganisms within a biofilm has been associated with a number of chronic infections. P. aeruginosa forms biofilms in the lungs of patients with cystic fibrosis (CF) and, despite the aggressive use of antibiotics, colonization is often a life-long problem, leading to chronic inflammation and lung tissue damage. Biofilm-forming P. aeruginosa also has a role to play in the persistence of cutaneous wound infections and has been shown to form biofilms in both human and veterinary wounds (Figure 5). In particular, chronic venous leg ulcers have often been shown to harbour P. aeruginosa, with P. aeruginosa-infected chronic wounds appearing larger than P. aeruginosa-negative wounds.
Using a specific P. aeruginosa PNA fluorescence in situ hybridization probe, the bacteria are visualized in red, whereas the inflammatory cells surrounding the biofilm patches are counterstained with 4’, 6-diamidino-2- phenylindole, DAPI (blue).23,24
The association of biofilms and medical device-related infections was first recognized in 1972, biofilms being commonly associated with a wide range of polymeric medical devices, such as catheters and cardiac pacemakers. The emergence of biofilm-related infections due to the widespread use of medical devices in healthcare settings (Figure 6) has given rise to the term ‘polymer-associated infection’.4
Scanning electron microscopic picture of a developing biofilm on urethral stents that is lying completely within the urinary tract.25
Antimicrobial resistance
Biofilms are formed by a spectrum of microorganisms, including pathogens, and provide a means for these organisms to protect themselves against antimicrobial agents.
Delayed penetration of the antimicrobial
For nutrient and antimicrobial molecules to reach microbial cells within biofilms, they must diffuse through the biofilm matrix or slime produced by and encasing the organisms. This diffusional limitation may be the result of either transport limitation (the inability of the antimicrobial molecules to diffuse through the polymer matrix) or inactivation of the antimicrobial molecule by the matrix material.3
Alteration of cellular growth rate
An alternative proposed mechanism for resistance of biofilm-associated cells (sessile organisms) to antimicrobials is that the growth rate of these organisms is significantly slower than the growth of planktonic (biofilm free) cells; therefore, the uptake of the antimicrobial molecules is diminished. Biofilm age may be important because with increasing age, there will be greater production of extracellular polymeric substances, leading to reduced nutrient and oxygen penetration into the biofilm matrix. It has also been suggested that phenotypic variants commonly referred to as ‘persister cells’ confer resistance within the biofilm owing to their slow rate of growth.3
Other hypothesis
Other mechanisms that are thought to play a role in the antimicrobial resistance acquired by certain micro-organisms within biofilms include the presence of efflux pumps, with the expression of several gene-encoding efflux pumps being increased in biofilms. Furthermore, plasmid exchange occurs at a higher rate in biofilms, increasing the chances of developing naturally occurring and antimicrobial-induced resistance. Finally, it is thought that an altered microenvironment within a biofilm, such as nutrient depletion and reduced oxygen levels, may also reduce the efficacy of antimicrobials.3
Virulence factors
Enterococcal infections are one of the most important global health problems causing considerable morbidity in the general population. Most of the enterococcal strains harboring virulence genes including, esp, cylA, and asa1, have been associated with human infections. Often these genes are located on specified region of the genomes, distinctively marked as “Pathogenicity Island”. Esp helps in adherence to the bladder wall via mucin and uroplakin receptors thus helping enterococcal colonization and persistence in urinary tract. Similarly, asa1 has been seen to help in adherence to renal cells.3 Though cytolysin expression is concurrent with hemolysin production, different components of the cytolysin operon containing five genes (cyl1, cyl2, cylA, cylM, and cylB) have been attributed to this hemolysis.26
Research is needed into cell signaling molecules, which are involved in quorum sensing and quorum quenching, and how they regulate biofilm formation and at what stage. Further the understanding of microbial interaction with biotic and abiotic surfaces and of how the adverse environment of the host affects microbial survival, proliferation and recalcitrance is required, to develop effective and broad spectrum anti-biofilm agents. The relevance of certain genetic determinants required for biofilm formation in vitro and these findings in vivo, using appropriate animal models that mimic the complex interaction between biofilm and host, is necessary to understand and to develop anti-biofilm agent.
None.
The authors declare that there is no conflict of interest.

©2018 Dewasthale, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.