International Journal of
eISSN: 2573-2889


Case Report Volume 4 Issue 3
1Head of Laboratory, Microbiologist and Clinical Chemistry and student of the Specialty Postgraduate in Medical Bacteriology, University of Costa Rica, Costa Rica
2Department of Microbiologist and Clinical Chemistry, Childrens National Hospital, Costa Rica
3Microbiologist and Clinical Chemistry and Specialist of Hematology at the University of Costa Rica, San Carlos Hospital, Costa Rica
4Head of Immunology Section, Microbiologist and Clinical Chemistry San Carlos Hospital, Costa Rica
5Head of Bacteriology Section, Microbiologist and Clinical Chemistry, student of the Specialty Postgraduate in Medical Bacteriology, University of Costa Rica, Costa Rica
6Microbiologist and Clinical Chemistry, San Carlos Hospital, Costa Rica
Correspondence: Gabriela Cruz-Chavarría , Head of Bacteriology Section, San Carlos Hospital, Alajuela, Costa Rica. Microbiologist and Clinical Chemistry, student of the Specialty Postgraduate in Medical Bacteriology at the University of Costa Rica
Received: April 29, 2019 | Published: May 6, 2019
Citation: Fallas-Mora A, Cordero-Montero I, Sánchez-Barahona M, et al. Diagnosis and follow-up of a Plasmodium vivax malaria case through the Sysmex XT1800i® hematology analyzer. Int J Mol Biol Open Access. 2019;4(3):80–83. DOI: 10.15406/ijmboa.2019.04.00101
Malaria is one of the most important parasitic diseases in the world. It is caused by parasites of the genus Plasmodium sp., which enter humans through the bite of mosquitoes of the genus Anopheles. Automated hematology analyzers based on flow cytometry have been widely used to aid in the diagnosis of malaria, based on the existence of alterations in the scatter plots that suggest the presence of parasites of the genus Plasmodium. The case of a 55-year-old male patient is presented, worker from a Costa Rican rural zone, correlating the alterations in channel DIFF and WBC/BASO of the Sysmex XT-1800i® hematological analyzer with what was found in the thick drop. The objective of this case report is to demonstrate the utility of hematological analyzers in the detection of relapses, reinfections and follow-up in patients diagnosed with malaria.
Keywords: paludismo, anopheles, plasmodium, p. vivax, malaria, Sysmex XT-1800i
Malaria is one of the most important parasitic diseases in the world. It is caused by parasites of the genus Plasmodium, which enter the human being through the bite of mosquitoes of the genus Anopheles. International efforts have been made in order to reduce the cases and deaths caused by the parasitosis; nevertheless, the WHO points out in the global report on malaria in 2018 that the scope has not been as expected, according to the agreements set in the Global Technical Strategy against Malaria 2016-2030. In 2017, 219 million cases of malaria were estimated worldwide, data from the period 2015 to 2017 highlight that no significant progress was made in reducing malaria cases within this period.1
The timely detection of malaria is crucial to control the survival of patients and the epidemiological control of the disease. Several Point of Care tests have been developed for the diagnosis of malaria and its use is focused in endemic areas. However, the effectiveness of any detection method is subject to clinical suspicion and specific medical request. In countries that are in the process of eradicating the disease, such as Costa Rica, the detection of new cases depends on the health personnel who provide care to patients. The thick drop analysis remains to be the most relevant standard for the diagnosis of malaria. The application of the test depends on the approach and clinical exploration of the patient that makes the health personnel suspect of the disease. The main limitation of this strategy is the variable presentation of signs and symptoms, being fever the main sign, which in turn is presented in innumerable differential diagnoses of first choice. In addition, the low incidence of the parasitosis can contribute to it to remain unnoticed. Studies of clinical cases in non-endemic areas indicate that in the first presentation the diagnosis is not made and, in some cases, the patient has had more than three medical visits prior to asking for the thick drop.2
In the last two decades, the automated hematological analyzers have been studied extensively based on flow cytometry for the diagnosis of malaria. Although these devices do not specifically detect parasites, they have documented in the scatter plots the existence of alterations that suggest the presence of parasites from the Plasmodium genus.3 The diagnostic sensitivity achieved with this analysis complies with the guidelines established by the WHO of≥95% in samples with>100 parasites/μL3. That is why the detailed analysis of dispersograms as part of the complete blood count can become an important tool for diagnosis with special attention to febrile patients, since these cases are usually sent to the laboratory for the realization of a blood count as part of their initial clinical exploration.
The XE-2100® analyzer has been used as a representative of the Sysmex commercial house for most malaria detection studies. In Costa Rica, the automated equipment distributed in Caja Costarricense del Seguro Social belongs to the XT series. The XT-1800i® and XT-2000i® models work under similar methodology to the first one, so that most of the described findings are compatible with the analyzers described in the literature. The objective of this case report is to demonstrate the utility of hematological analyzers in the detection of relapses, re infections and follow-up in patients diagnosed with malaria.
It is presented the case of a 55-year-old male patient, worker from a banana zone in the rural countryside of Costa Rica near the northern border with Nicaragua. The patient approaches to the health center complaining for chills, fever, excessive sweating, headache, lack of appetite, leg cramps and intense abdominal pain of a month of evolution. The laboratory is ordered to perform a thick drop, finding parasitic forms of the genus Plasmodium in the extension. The patient is referred to the health center corresponding to his zone to make the definitive diagnosis by species and to initiate the pharmacological treatment.
In the first report of the hematological analyzer (Sysmex XT-1800i®), the results are obtained for hemoglobin, hematocrit and normal hematimetric indexes, normal leukocyte formula and mild decrease in platelets (Figure 1). In the DIFF channel, the populations of eosinophils and neutrophils are observed as a single population in light blue. In the WBC/BASO channel, blue sky events are observed grouped in the third quadrant.
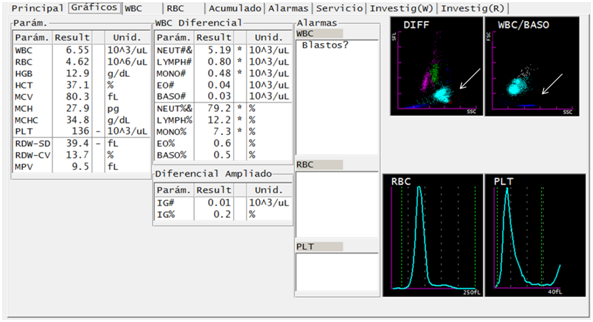
Figure 1 Figure 1 Suggestive traits of parasites of the genus Plasmodium in a case of Plasmodium vivax infection, through DIFF and WBC/BASO graphics generated from the hematological analyzer Sysmex XT-1800i ©. The scatter plot DIFF (SSC versus SFL) shows lymphocytes (magenta), monocytes (green), neutrophils (light blue), eosinophils (red) and RBC phantoms (blue). The malaria-related abnormality it presents is the fusion of the neutrophil and eosinophil groups (arrows). The WBC/BASO scatter plot (SSC versus FSC) separates WBCs (sky blue) from basophils (gray). The finding related to malaria is in the quadrant (III), there is presence of blue sky events (arrow).
In the blood smear and thick drop, trophozoites, schizonts and gametocytes were observed. In accordance with national regulations, a whole blood sample was referred to Centro Nacional de Referencia en Parasitología (National Reference Center in Parasitology). It was analyzed by Real time TaqMan® PCR with probes for P. vivax, P. falciparum and P. malariae, being positive for P. vivax, only. The diagnostic confirmation was complemented with thick drop. A total of 5243 asexual blood stages+175 sexual blood stages including gametocytes, schizonts and trophozoites were reported (Figure 2).
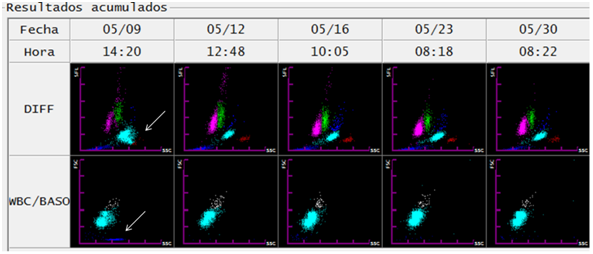
Figure 2 Follow-up of a patient with malaria infection by Plasmodium vivax through evolutionary graphs of DIFF and WBC/BASO generated from the hematological analyzer Sysmex XT-1800i ©. On the day of diagnosis (1) the abnormality related to malaria (arrows) is observed in the DIFF scatter chart. On the other hand, the WBC/BASO dispersion chart (SSC versus FSC): shows the finding related to malaria in the quadrant (III), presence of events is observed (sky blue) (arrow). In the following days of starting of treatment (day 1), both findings disappear, thus observing DIFF scatterplots with defined cell populations (lymphocytes (magenta), monocytes (green), neutrophils (light blue), eosinophils (red)).
Before the laboratory diagnosis, the patient received chloroquine and primaquine in a supervised manner. He underwent parasitaemia control at 3, 5, 12, 19 and 28 days after thick drop treatment according to regulations, analysis was complemented with complete hemogram. Sexual blood stages presence was obtained only on the day of diagnosis. On the third day, the asexual blood stages were reduced from 5243 to 245 with 0 sexual blood stages. By the fifth day and until the end of the follow-up, no parasitic forms were observed. The patient responded satisfactorily from the third day without fever and showed favorable tolerance to treatment.
Six months after treatment, the patient returned to the health care center with recurrence of fever and headache. Following his recent history of malaria, laboratory analyzes were ordered in this context. A blood count was performed with the Sysmex XT-1800i® hematology analyzer (Figure 3). In the DIFF channel, proximity to overlap was observed in populations of eosinophils and neutrophils, in this case the representation of both populations was maintained in red and light blue, respectively. On the WBC/BASO channel, sky blue events reappeared grouped in the third quadrant. In addition, 3694 asexual blood stages and 295 sexual blood stages of P. vivax are observed in the smear and thick drop. Failures to treatment or reinfection are the two possible scenarios considering the previous findings.
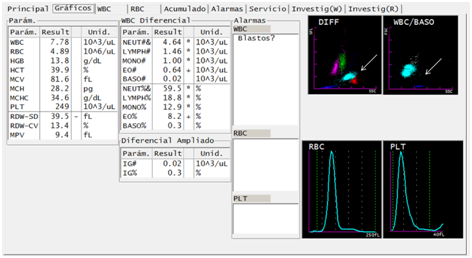
Figure 3 Reappearance of suggestive findings of parasites of the Plasmodium genus in a patient treated for malaria infection by Plasmodium vivax, through DIFF and WBC/BASO graphics generated from the Sysmex XT-1800i © hematological analyzer. After six months of the diagnosis and concluded the pharmacological treatment, the patient presents to the Health Care Center with febrile symptoms. The sample taken again shows the abnormality described as fusion of the neutrophil and eosinophil groups (arrow). The WBC/BASO scatter plot presents the finding related to malaria in the quadrant (III), described as light blue events (arrow).
Multiple studies have defined a pattern of scatter plots in samples of patients with malaria. The following findings are included in the DIFF Channel.3–5
Fusion of populations of eosinophils and neutrophils, which may be red, sky blue or gray.
Appearance of multiple groups of eosinophil and neutrophils.
Prominent events in blue in the region of red blood ghosts cells below the neutrophil and/or eosinophil group.
Sum of the patterns described in points 1 and 3.
Irregular form of the group of events denoted by neutrophils.
Pseudoeosinophilia.
In the WBC/BASO Channel it is included6
Events highlighted in blue in the third quadrant
It is important to point out that these alterations have not been found in samples of febrile patients without malaria. On the other hand, the described patterns are more sensitive to detect P. vivax, than P. falciparum and other species.5,6 The patterns described are not related to the parasitic index but to the presence of trophozoites, schizonts or ring forms. A higher number of mature trophozoites and schizonts correlates with anomalies in the DIFF channel.4–6 In other studies, the sensitivity of the method has been subject to the number of young parasites in circulation, the severity of the infection and the patient's immune response, which limits the production of hemozoin staying below the detectable limits of the analyzer.4 In the case described, despite having low parasitic density, the presence of mature trophozoites and schizonts could make perceptible the finding in the hematological analyzer.
The abnormalities of the DIFF channel are due to neutrophils that contain hemozoin, by definition the waste product from blood’s digestion carried out by some parasites.3 This pigment has high properties of lateral dispersion, so that this property increases in neutrophils, causing the observed overlap with the population of eosinophils. On the other hand, events encoded in blue in the third quadrant of the WBC/BASO channel have been associated with erythrocytes and reticulocytes infected with Plasmodium vivax. As all forms of parasites are present in P. vivax infection, contrary to what was observed with P. falciparum, the events highlighted with blue are seen more frequently in the first.7 It is important to clarify that these patterns only suggest the presence of parasites of the genus Plasmodium, it is necessary to resort to confirmatory techniques to establish a diagnosis.
In the case described, an evolutionary record of the dispersograms with the hematological analyzer (Sysmex XT-1800i®) was obtained during the first 19 days after the diagnosis (Figure 2). The disappearance of events in sky blue in the third quadrant of the WBC/BASO channel coincided with the clinical remission evidenced by the patient after the application of the treatment. In the DIFF channel, the proximity and overlap observed in populations of eosinophils and neutrophils disappeared. As shown in Figure 2, both changes detected from the first control blood count were maintained for the following 16 days.
The DIFF channel generated from the cell population at 6 months post treatment (Figure 3) shows the dispersion pattern similar to that described in the literature as pseudoeosinophilia.5,6 Retrospective cohort studies have indicated that patients infected with P. vivax have falsely elevated eosinophil counts. In the DIFF channel, it is noticed events with scattering in the specific magnitude that the analyzer classifies as eosinophils.6,7 Pseudoeosinophilia does not provide conclusive information as an isolated value, the complementary analysis of the events in quadrant (III) of the WBC/BASO chart generates enough elements to justify the performance of confirmatory tests such as thick drop, even more in the context of the febrile patient.
On the other hand, infection by P. vivax is characterized by formation of hypnozoites with permanence in host hepatocytes; this phenomenon is the main explanation for relapses. Primaquine schizontocide constitutes the radical treatment2 for these parasitic stages. The therapeutic failures are associated with suboptimal dosages, problems of adherence and reinfections; these factors are not presented by the patient of the case, which proposes some mechanism of their own metabolism and tolerance of the parasite to the immune system.
Many reports of infections by parasites of the Plasmodium genus have been detected incidentally when analyzing routine hematological samples. Cases have been diagnosed where there is no clinical suspicion, but dispersogram analysis generates alarm from the clinical laboratory.8,9 Proper training of laboratory personnel responsible for analyzing the DIFF and WBC/BASO charts can allow them to recognize changes related to malaria and complement the finding with diagnostic tests. Currently, the participation of the industry is fundamental for the development of warnings that guide the microscopic evaluation of the patient's sample. The developers of the Sysmex® line have recently focused on the evaluation of algorithms that can generate warnings with high suspicion of malaria. In this way, it is expected to guide all analysts towards the diagnosis, since currently the recognition of the described patterns can be achieved only in experienced analysts.
Costa Rica, being a non-endemic area in the process of eradication of malaria, should remain alert and monitor the appearance of isolated cases. In a laboratory environment, where the diagnosis of the disease can go unnoticed initially, the microbiologist must recognize suggestive patterns so that he does not miss the opportunity to capture cases. In addition, the wide distribution of automated hematology analyzers in the country should be taken advantage of, associated with prescriptions of blood counts as part of the initial approach to febrile symptoms. In this line, the detailed analysis of dispersograms represents an important intervention opportunity in cases whose prognosis depends on the early diagnosis.
None.
The Authors declare no conflicts of interests.

©2019 Fallas-Mora, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.