International Journal of
eISSN: 2381-1803


Research Article Volume 10 Issue 2
1Department of Pharmacy, Federal University of Ceará - UFC, Brazil
2Department of Pharmacy, University of Fortaleza- UNIFOR, Brazil
Correspondence: Kalyne Almeida Moreira Leal, CEFAC, Centro de Estudos Farmaceuticos e Cosm, Brazil, Tel 55 85 3366-8249
Received: July 26, 2017 | Published: December 27, 2017
Citation: Louchard BO, Costa LC, Silva ARA, Leal LKAM (2017) Validation of A High Performance Liquid Chromatography Method to Quantify Thymol in Nanocapsules of Bioactive Essential Oil from Lippia Sidoides . Int J Complement Alt Med 10(2): 00330. DOI: 10.15406/ijcam.2017.10.00330
The essential oil of L. sidoides is a derivative of a natural product with antimicrobial antioxidade and has great potential for medical use. The objective of this study was to validate a method for HPLC–DAD for quantification of thymol, an important compound of essential oil in nanocapsules containing the essential oil of L. sidoides. The method was linear in the range of 4–180μg mL–1, with a good correlation coefficient (r=0.9994). Precision and accuracy analysis showed low relative standard deviation (<2.27 %) and a good recovery percentage (97.2–98.2 %). The procedure proved to be specific, linear, precise, exact and robust so that the method can be applied in quantification of thymol in suspensions of nanocapsules containing the essential oil of L. sidoides.
Keywords: validation, lippia sidoides, thymol, nanocapsules, essential oil, hplc–dad
ANVISA, Agência Nacional De Vigilância Sanitária (National Health Surveillance Agency Brazil); ICH, Conference of Harmonization; GC–MS, Gas Chromatography–Mass Spectrometry; LC–MS, Liquid Chromatography–Mass Spectrometry; LOQ, Quantification; LOD Limits of Detection; HPLC–UV, High Performance Liquid Chromatography And Ultravioleta Detector; HPLC–DAD, High Performance Liquid Chromatography and Diode Array Detector; NCB, Blank Nanocapsules; NCLS, Nanocapsules Containing Essential Oil of L. Sidoides; ODS, Octadecylsilyl Groups; OELS, Essential Oil of Lippia Sidoides; PLGA, Poly D, L–Lactide–Co–Glycolyde; PTFE, Polytetrafluoroethylene; R.S.D, Relative Standard Deviation
Lippia sidoides Cham. (Verbenaceae), popularly known as rosemary pepper, is an aromatic shrub native to the Brazilian Northeast.1 The essential oil extracted from its leaves has a high concentration of thymol, a component of potent antimicrobial activity. Studies have reported that the essential oil of L. sidoides has several biological properties including antimicrobial.2,3 antifungal.4,5 antioxidant.6,7 and topical anti–inflammatory.7,8 However, some studies report irritant action of the mucosa when the essential oil is applied directly to the skin.9 Thus the essential oil of L. sidoides was encapsulated in polymeric nanocapsules in order to decrease the toxic effects and maintain or increase its therapeutic efficiency, because this is one of the purposes of nanoencapsulation.10 High–performance liquid chromatography is one of the quantification techniques commonly used for drug analysis in the pharmaceutical industry, and has been a widely used methodology in the analysis of conveyed drugs in nanocarriers.11–13 For essential oils, gas chromatography analysis techniques are generally employed, however, thymol, which is the major component and the pharmacological marker in L. sidoides essential oil, has an absorption spectra in the ultraviolet region. This fact allows the quantification of thymol in essential oils, plant extracts and nanocarriers by HPLC–UV and HPLC–DAD, as has been reported in several studies.14–17 Therefore, the aim of this study was to validate an analytical methodology by HPLC–DAD, for quantification of thymol in essential oil and nanocapsules containing essential oil of L. sidoides.
Reagents, solvents and materials
Thymol (99.5 % purity), PLGA (Poly D, L–lactide–co–Glycolyde 50:50) and poloxamer 188 (Poly(ethylene glycol)–block–poly(propylene glycol)–block–poly(ethylene glycol) were purchased from Sigma Aldrich (São Paulo, USA), acetonitrile grade HPLC and acetone grade P.A. were purchased from Tedia (New Janeiro BR), water purified through milli–q filter system (Milipore, Brazil), phosphatidylcholine (Lipoid S–100) were purchased from Lipoid (Newark, USA), essential oil of Lippia sidoides (OELS) was purchased from Padetec (Fortaleza, BR), Millex–LCR filter with PTFE membrane modif. 0,45μm was purchased from Milipore (São Paulo, BR).
Instrumentation
The chromatographic analyzes were performed on a liquid chromatography (Waters Alliance 2695, USA) with diode array detector (Waters Alliance 2996, USA). Analytical separation was achieved on a reversed phase C18 column ODS Hypersil (Thermo 250 mm x 4.6 mm, 5µm particle size), coupled to a C18 security guard pre–column (Thermo 10 x 4mm). A rotary evaporator (Heidolph G3, Germany) and magnetic stirring (Quimis 601.2, Brasil) were used.
Preparation of nanocapsules
Nanocapsules containing essential oil of L. sidoides (NCLS – 7,5mg mL–1 of OELS) (n = 3 batches) and blank nanocapsules (NCB) were obtained by nanoprecipitation of preformed polymer.18 PLGA (36mg), phosphatidylcholine (90mg) and (0.01g) were dissolved in acetone (15mL) at 40 °C±2. This organic phase was injected into an aqueous phase at 40 °C±2, containing poloxamer 188 (180 mg) and ultrapurified water (30mL) under magnetic stirring. After 10 min, acetone was eliminated and the suspension concentrated to 10mL under reduced pressure (rotary evaporator). Blank nanocapsules were prepared in the same manner, but the OELS was not added to the organic phase.
Chromatographic parameters
The chromatographic conditions were: column temperature 40 °C, mobile phase acetonitrile: water (78 : 22), isocratic elution mode, flow 0.8ml min–1, detection wavelength 278nm, sample injected volume 20μL, and run time 8 min. Before delivering the mobile phase into the system, it was degassed for 30 min by sonication and filtered through 0.45μm filter using vacuum. These experimental conditions were established on a method previously developed by our research group for the quantification of thymol in leaf extracts of L. sidoides.14
Preparation of standard solutions
The amount of 10 mg of thymol reference standard was transferred to a 10mL volumetric flask and the volume was completed with the mobile phase. From this, thymol stock solution (1 mg mL–1) was prepared by 8 dilutions for construction of the calibration curve (8 µg mL–1, 10 µg mL–1, 20 µg mL–1, 30 µg mL–1, 50 µg mL–1, 100 µg mL–1, 150 µg mL–1, 180 µg mL–1).
Preparation of samples solutions
OELS samples were prepared by weighing approximately 20mg OELS directly into a 10 ml volumetric flask using an analytical balance (Toledo, Brazil), the volume was completed with the mobile phase. From this solution (2mg ml–1 OELS), dilutions were prepared 16 – 24µg ml–1 OELS as required for the test. The samples NCLS or NCB were prepared by adding 80–120μL of the sample in a volumetric flask of 10 ml and completing the volume with mobile phase. The standard and sample solutions were always prepared on the day of analysis, filtered through a 0.45μm membrane (Millipore, USA) and transferred to a vial for injection equipment.
Method validation
The method was validated according to the International Conference of Harmonization (ICH) and the ANVISA Resolution RE 899/2003 guideline.19 considering the following parameters: specificity, linearity, precision, repeatability, accuracy, recovery, limit of quantification, limit of detection and robustness.
Robustness
The robustness of the method was assessed by varying the parameters of the column temperature, flow rate of mobile phase and column manufacturer, as described in Table 1. Samples corresponding to 100 % of the theoretical test concentration corresponding to 20µg ml–1 of OELS were evaluated. The results were expressed as percentage of 100 % nominal content where content corresponded to 600 mg of thymol in each mL of OELS. Statistical analysis was by the ANOVA analysis of variance followed by Tukey's test, the results were considered significant when p <0.05.
|
Standard Method |
Challenging Conditions |
|||
|
1 |
2 |
3 |
||
|
Temperature |
40 °C |
30 °C |
40 °C |
40 °C |
|
Column supplier |
Termo |
Thermo |
Fenomenex |
Thermo |
|
Flow (ml min-1) |
0.8 |
0.8 |
0.8 |
1 |
Table 1 Parameters evaluated in verifying the robustness
The selectivity or specificity is considered the first step of validating analytical methods, it is through this study that it can be inferred that the components of the matrix or excipients do not interfere with the quantification of the drug.21. The specificity of the methodology related to testing showed that the method was capable of identifying the component of thymol retention time at 4.95 min for both OELS and NCLS (Figure 1). The chromatographic analysis showed that the NCB excipients that make up the matrix of the nanocapsules thymol did not interfere with the analysis by this method since there was no appearance of other peaks in the chromatogram. Therefore, we can conclude that the proposed method is specific for quantification of thymol in NCLS. The analysis of the linearity parameter provides information about the ability of the method to provide results that are proportional to the concentration of analyte in the sample.22
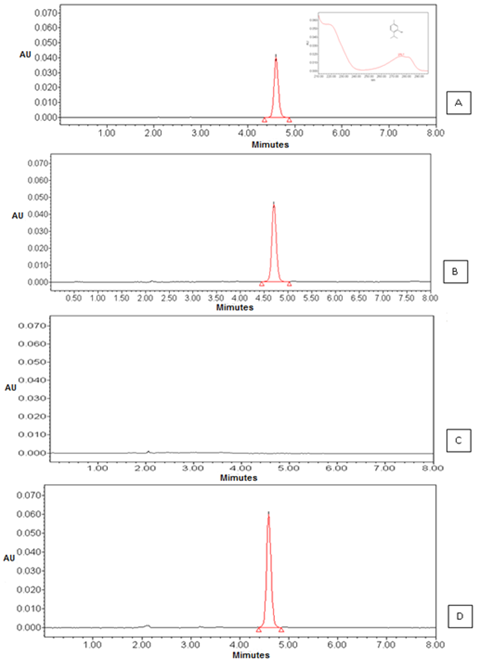
Figure 1 Chromatograms corresponding to: (A) the peak thymol and UV spectrum (B) the peak thymol in OELS viewed by HPLC-DAD observed at 4.9 minutes (C) blank nanocapsules and (D) the peak thymol in nanocapsules.
The linearity was studied by analysis of variance, calculating the linear regression by the method of least squares by using a curve consisting of 5 concentrations in triplicate, the OELS and NCLS within the concentration range set for thymol (4 –180µg mL–1). The analytical curves in triplicate with seven standard concentrations of thymol showed a correlation coefficient (r) of 0.9994, the equation of the straight line obtained was of the type y = a. x + b where y is the peak area which is a linear function of the concentration of the substance "x". Figure 2 graphically demonstrates the analytical curve of thymol and the equation of the corresponding y = 24592 x – 19984.
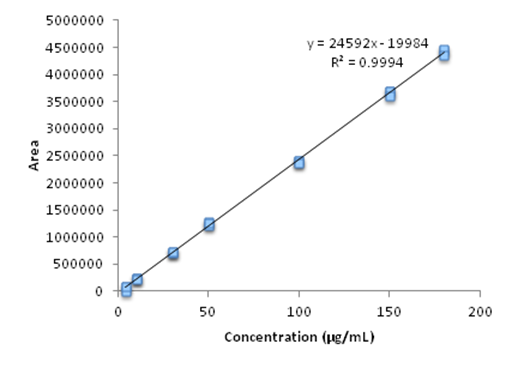
Figure 2 Graphical representation of the calibration curve obtained by HPLC-DAD, referring to seven concentrations of thymol (4 - 180μg ml-1) evaluated in the study of method linearity.
The curves of the five concentrations of oils and NCLS evaluated showed a correlation coefficient of (r) 0.9991 and 0.9979 respectively. An important factor in evaluating the method of linearity is the analysis of waste graph. Through this analysis it is possible to detect problems in curve fitting, such as linearity deviation, presence of atypical sample heteroscedasticity, and dependence between the errors. So a well–adjusted curve must have errors with uniform distribution, zero mean and constant variance (homoscedasticity), as well as the absence of atypical strains.20. As can be seen in Figure 3 relating to waste curves obtained with the OELS and the NCLS, they proved to be normally and independently distributed around zero, demonstrating the absence of waste there between.
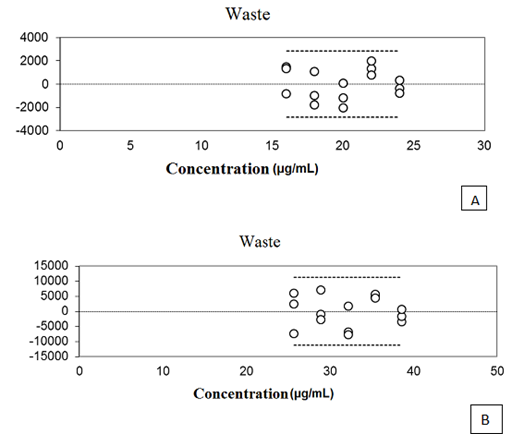
Figure 3 The graphical representation of waste relating to the five concentrations of OELS (A) and NCLS (B).
According to Ribeiro et al.20 waste represents the portion of data variability that is not explained by the adjustment, and can be interpreted as an estimate of the model error. In this way, the graphical representation of waste must be normally and independently distributed around zero, and must have zero mean and constant variance. In the present study, the waste graphic pattern regarding thymol, OELS and the NCLS showed this behavior. The analysis of variance (ANOVA) conducted for the calibration curve of thymol (P = 0.57), OELS (P = 0.39) and NCLS (P = 0.20) demonstrated that the present method does not present a significant linearity deviation. Thus we can infer that the developed method has linear response to the detector of the thymol in the range of 4 – 180μg mL–1. Statistical analysis of the data relating to the linearity of the method allowed the calculation of the limit of detection (LOD) and the limit of quantification (LOQ) through a reliable calibration curve range. The limit of detection for thymol assay was 0.4μg ml–1 and the quantitation limit was 1.02μg mL–1. The analytical method should ensure a reliable analysis of the drug to be quantified, so it must be accurate, and present minimum dispersion between the results of the readings at the same concentration and at the same time be accurate, which means that there must be agreement between individual results in the same test or self–test, compared to a reference value accepted as true.21 The accuracy test evaluated the concordance of results at repeatability levels (within–run precision) and intermediate precision (inter–run precision). The OELS and NCLS showed RSD values of 1.05 and 2.22 respectively for repeatability at 100 % of theoretical test concentration (Tables 2 & 3). In the evaluation of intermediate precision, the RSD values for OELS ranged from 0.62 to 3.06, and for the NCLS the values ranged from 0.34 to 3.71 (Tables 4 & 5). The F test (ANOVA) applied to evaluate the homogeneity of variance in the intermediate accuracy test showed no significant differences between the analyses carried out by different analysts or on different days (p> 0.05) for both OELS and NCLS, as can be seen in Tables 6 & 7. Therefore from the results we can infer that the method is accurate.
|
Concentration |
Content % |
Average |
RSD |
|
101.1 |
100.5 |
1.05 |
|
|
99.7 |
|||
|
101.6 |
|||
|
101.1 |
|||
|
98.8 |
|||
|
100.8 |
|||
|
100% |
Table 2 Thymol quantification results in OELS performed in a single day regarding the repeatability test (within-run precision)
Results are expressed as nominal content of thymol in OELS, referring to the mean ± standard deviation of three replicas, where 100% of content is equivalent to 600mg of thymol per 1mL of OELS.
|
Concentration |
Content % |
Average |
RSD% |
|
100%
|
99.4 |
99.5
|
2.22
|
|
95.6 |
|||
|
99.4 |
|||
|
102.4 |
|||
|
100.5 |
|||
|
99.6 |
Table 3 Thymol quantification results in NCLS performed in a single day regarding the repeatability test (within-run precision)
Results are expressed as nominal content of thymol in NCLS concerning the mean ± standard deviation of three replicates, where 100% of theory is equivalent on average to 3.2mg thymol per 1 ml NCLS.
|
Day |
Analyst |
Concentration |
|||||
|
|
|
80% |
100% |
120% |
|||
|
Content (%) |
RSD % |
Content (%) |
RSD % |
Content (%) |
RSD % |
||
|
1 |
I |
100.4 |
0.62 |
99.8 |
3.06 |
100.6 |
1.27 |
|
II |
98.4 |
0.34 |
99.7 |
1.8 |
100.8 |
1.98 |
|
|
2 |
I |
100.9 |
1.06 |
100 |
0.34 |
99.4 |
1.34 |
Table 4 Quantification of thymol in OELS
Results are expressed as nominal content of thymol in OELS, referring to the mean ± standard deviation of three replicas, where 100 % of content is equivalent to 600 mg of thymol per 1mL of OELS. Results for the intermediate precision test (inter run precision).
|
Day |
Analyst |
Concentration |
|||||
|
|
|
80% |
100% |
120% |
|||
|
Content (%) |
RSD % |
Content (%) |
RSD % |
Content (%) |
RSD % |
||
|
1 |
I |
103.4 |
0.62 |
99.8 |
3.06 |
100.6 |
1.27 |
|
II |
98.4 |
0.34 |
99.7 |
1.8 |
100.8 |
1.98 |
|
|
2 |
I |
100.9 |
1.06 |
100 |
0.34 |
99.4 |
1.34 |
|
Dia |
Analyst |
Concentration |
|||||
|
|
80% |
100% |
120% |
||||
|
Content (%) |
RSD % |
Content (%) |
RSD % |
Content (%) |
RSD % |
||
|
1 |
I |
103.4 |
2.18 |
98.9 |
1.34 |
99.4 |
0.34 |
|
II |
102.8 |
1.68 |
95.5 |
3.71 |
102.4 |
1.42 |
|
|
2 |
I |
103.2 |
1.62 |
99.5 |
2.1 |
101.9 |
2.27 |
Table 5 Quantification of active thymol in NCCLS. Results for the intermediate precision test (inter run precision)
Results are expressed as nominal content of thymol in NCLS concerning the mean ± standard deviation of three replicates, where 100 % of theory is equivalent on average to 3.2mg thymol per 1ml NCLS.
|
Experimental condition |
Concentration |
||||||
|
80% |
100% |
120% |
Fcrit |
||||
|
F |
P |
F |
P |
F |
P |
||
|
Day 1 analyst I versus day 1 analyst II |
9.512 |
0.091 |
0.003 |
0.961 |
0.027 |
0.885 |
18.513 |
|
Day 1 analyst I versus day 2 analyst I |
0.108 |
0.773 |
0.076 |
0.808 |
0.977 |
0.427 |
|
Table 6 Statistical evaluation of results for the intermediate precision test for OELS
|
Experimental condition |
Concentration |
||||||
|
80% |
100% |
120% |
Fcrit |
||||
|
F |
P |
F |
P |
F |
P |
||
|
Day 1 analyst I versus day 1 analyst II |
1.31 |
0.37 |
0.72 |
0.48 |
4.25 |
0.17 |
18.51 |
|
Day 1 analyst I versus day 2 analyst I |
0.66 |
0.49 |
6.36 |
0.12 |
1.36 |
0.36 |
|
Table 7 Statistical evaluation of results for the intermediate precision test for NCLS
The accuracy test is to prove the efficacy of the method for quantifying the analyte in the sample.22 According to Brito.23, the accuracy is expressed as a percentage of systematic error inherent in the process. This systematic error may occur due to low recovery of the analyte in the extraction process, inaccurate volumetric measurements or interfering substances in the sample. The accuracy of analysis results conducted at three concentration levels for OELS and the NCLS are expressed in Table 8. As can be seen the method had a recovery of around 97.24 % with RSD of 1.04 for the quantification of thymol in OELS and a recovery of 98.2 % with RSD of 1.98 for NCLS.
|
Concentration |
OELS |
NCLS |
||||
|
Recovery (%)* |
Average (%) |
RSD% |
Recovery (%)* |
Average (%) |
RSD% |
|
|
10µg mL-1 |
96.1 ± 0,05 |
99.9 ± 3.81 |
||||
|
50µg mL-1 |
97.6 ± 1,23 |
97.24 |
1.04 |
98.6 ± 1.60 |
98.2 |
1.98 |
|
100µg mL-1 |
98.0 ± 2.13 |
96.1 ± 2.99 |
||||
Table 8 Results of the recovery method for thymol assay in OELS and NCLS.
* Results of the mean ± standard deviation of 3 determinations
The robustness of the method was assessed by varying the temperature parameters of the column, the mobile phase flow and the column manufacturer. As can be seen in Table 9, none of the parameters significantly impact the dosing of the active thymol (P = 0.3929). The robustness test identifies the method of factors that have a significant effect on the results and anticipate problems that may arise during the application of the method on different instruments, using different reagents, chromatographic columns, and in different environments.24 Therefore, from the results presented we can infer that the developed method can be considered robust for the tested parameters.
|
Condition standard method |
Temperature |
Column |
Flow |
|
30 °C |
Fenomenex |
1,0 mL min-1 |
|
|
Teor (%) |
|||
|
101.8 ± 0.96 |
99.3±2,87 |
98.3±2.14 |
101.8±4.47 |
Table 9 Influence of temperature, column provider and the mobile phase flow, the robustness of the measurement method of thymol.
The results of our study demonstrate the suitability of the method for quantification of thymol using HPLC–DAD. The method proved to be simple, rapid, specific, linear, accurate and robust for sensitive quantification of the active thymol in essential oil as well as in nanocapsules containing the essential oil of L. sidoides. The analytical procedure has a total analysis time of eight minutes which allows for the analysis of a large number of samples in a short time. Therefore, it is suitable for routine analysis of both raw material of OELS and the formulation of nanocapsules containing OELS. The simplicity of the method allows the for application in laboratories that do not have access to sophisticated analytical instruments such as LC–MS or GC–MS equipment, which need a more complicated, more costly and time consuming analysis.
This work was supported by the CAPES and CNPq.
The author declares that there is no conflict of interest.
None.

©2017 Louchard, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.