eISSN: 2471-0016


Glucans are known to have anti-inflammatory properties and several studies have demonstrated their beneficial effects on intestinal disorders. In our study, we used an established animal model of neonatal necrotizing enterocolitis to determine the potential effects of glucan on the development of this most common intestinal disease of prematurely born babies. We found significant decrease in the incidence of necrotizing enterocolitis in the glucan-treated group. Oral application of glucan affects intestinal expression of IL-18, TNF-a, iNOS, CXCL1, Tff3, and Muc-2, suggesting that glucan’s effects are manifested via reduction of inflammatory response at the site of injury.
Keywords: glucan, NEC, gut, intestine, inflammation
b(1-3)-D-Glucan’s (glucans) are natural products found in yeast, mushrooms, grains and seaweed. β-Glucans represent a group of heterogeneous polysaccharides (both chemically and physico-chemically) with strong immunomodulatory capabilities. Natural glucans are found mainly in whole grains, yeast, and mushroom. They represent a complex group of chemically heteregenous polysaccharides with significant immunomodulatory activities. Glucans are available in many dietary supplements; therefore, they can be easily used as complementary and alternative medicine. In addition to many animal studies where glucans were found to be active in a wide range of species (basically from shrimp to horses) the effects of glucans have been also examined in humans. Recently, a series of clinical studies showed strong effects on the treatment of children with chronic respiratory problems.1,2
The anti-infectious properties, ability to activate the immune system, and tumoricidal effects of β-glucans have been well known for decades (for review see.3 However, only a few recently published studies have tested the effect of glucans on the gastrointestinal (GI) tract.4,5 with particular interest in colitis.6–8 As the effects of glucans on the development of neonatal intestinal injury have not been studied yet, this study describes new effects of glucan. Neonatal necrotizing enterocolitis (NEC) represents the most common intestinal disease of prematurely born babies. As it is associated with excessive morbidity and mortality,9,10 it remains a serious problem. Current treatment strategies include introduction of broad-spectrum antibiotics, discontinuation of enteral feedings, or intravenous hyper-alimentation.9 Severe forms of NEC often require surgical intervention,11 resulting in the short-bowel syndrome in these patients causing life-long problems. Combination of a genetic predisposition, overreaction of intestinal mucosal tissue and intestinal immaturity seems to be the most probable cause.9 However, the pathogenesis and etiology of NEC are still not fully elucidated and no effective and lasting treatments are available. Therefore, the successful search for novel therapies and preventive treatments for NEC is of critical importance.
Based on our previous pioneering studies showing the absorption of orally administered glucan by healthy intestine of suckling rats,12 we decided to use a rat model of NEC to investigate the effect of glucan administration on NEC. Neonatal rat NEC model is an invaluable experimental tool to study pathogenesis and prevention of this disease13 and has been repeatedly used in search for novel treatments of NEC.14–16 This study is focused on determination whether oral supplementation with yeast-derived glucan will protect intestine against experimental NEC injury.
Animal model
Neonatal Sprague-Dawley rats (Charles River Laboratory, Pontage, MI, USA) were collected by caesarian section 24hr before their scheduled birth, and the first feeding started 2hrs. After delivery. Animals were hand fed six times daily with a total volume of 0.85ml of rat milk formula/day.17 Experimental NEC was induced by asphyxia (breathing 100% nitrogen gas for 60s) and cold stress (4°C for 10min) twice daily.14 Caesarian section-delivered pups were divided into the following experimental groups: neonatal rats formula fed (FF; n=26), neonatal rats fed with formula containing glucan (FF+Glucan; n=29), and dam-fed littermates fed by surrogate mothers as a baseline control (DF; n=7). After 96hrs, all surviving animals were terminated via decapitation. Animals that developed signs of distress or imminent death before 96 h were terminated and included in the study. The rat milk formula used for feeding of neonatal rats was prepared primarily from evaporated milk as described previously.14 In the FF group, the major source of lipids in the formula was the mixture of Intralipid (Fresenius Kabi, Uppsala, Sweden) and almond oil. In the FF+Glucan diet, almond oil was replaced with 1.5% wt/wt of glucan.
NEC evaluation
After termination, a 2-cm piece of distal ileum was removed and fixed in 70% ethanol, paraffin embedded, sectioned at 4-6μm, and stained with hematoxylin and eosin for histological evaluation of NEC. Pathological changes in intestinal architecture were evaluated using our previously published NEC scoring system.14 Histological changes in the ileum were scored by a blinded evaluator and graded as follows:0(normal), no damage; 1(mild), slight submucosal and/or lamina propria separation; 2(moderate), moderate separation of submucosa and/or lamina propria, and/or edema in submucosal and muscular layers; 3(severe), severe separation of submucosa and/or lamina propria, and/or severe edema in submucosa and muscular layers, region villous sloughing; 4(necrosis), loss of villi and necrosis. Intermediate scores of 0.5, 1.5, 2.5 and 3.5 were also utilized to more accurately assess levels of ileal damage when necessary. To determine incidence of NEC, animals with histological scores of <2 have not developed NEC; animals with histological scores of ≥2 have developed NEC.18
RNA preparation and real-time PCR
Total RNA was isolated from ileal tissue (snap frozen in liquid N2) using the RNeasy Plus Mini Kit (Qiagen, Santa Clarita, CA) as described in the manufacturer's protocol. RNA concentration was quantified by ultraviolet spectrophotometry at 260nm, and the purity and integrity were determined using a NanoDrop (Thermo Fisher Scientific, Wilmington, DE).19 RT real-time PCR assays were performed to quantify steady-state mRNA levels of selected molecules IL-12p35, TNF-β, iNOS, CXCL1, Tff3, and Muc-2.20 cDNA was synthesized from 0.2μg of total RNA. Real-time PCR amplification was performed using Primer Express Software (Applied Biosystems, Foster City, CA). Target probe was labeled with fluorescent reporter dye. PreDeveloped TaqMan primers and probes were used for the detection. Reporter dye emission was detected by an automated sequence detector combined with ABI Prism 7700 Sequence Detection System software (Applied Biosystems). Real-time PCR quantification was then performed using TaqMan 18S controls.
Immunohistology and enumeration of MUC2- and Tff3-positive cells.
Serial sections of the ileum were stained for either MUC2 or Tff3. Briefly, after deparaffinization and rehydration, sections were incubated with either rabbit anti-MUC2 polyclonal antibody (Santa Cruz Biotechnology) or rabbit polyclonal anti-Tff3 antibody21 for 30min, washed with PBS three times, and incubated with a biotinylated goat anti-rabbit secondary antibody (Vector Laboratories) for 30min. Vectastain Elite ABC reagent (Vector Laboratories) was then applied, followed by diaminobenzidine as a substrate. Sections were counterstained with hematoxylin, dehydrated, and mounted on coverslips. Muc2-and Tff3-positive cells were counted from each animal.
b-1,3 glucan
Yeast-derived, 85% pure insoluble Glucan #300 were purchased from Transfer Point (Columbia, SC).
Statistics
Statistical analyses between individual groups were performed using ANOVA followed by Fisher paired least-significant difference and by the Student's t-test at the 95% confidence level. Analysis of the NEC scores between groups was accomplished using the Kruskal-Wallis test for nonparametric values followed by pairwise comparison using the Mann-Whitney test. The Pearson's chi-squared (χ2) test was used to analyze differences in incidence of NEC between groups. All statistical analyses were conducted using the statistical program StatPlus:mac LE for Macintosh computers (AnalystSoft, Alexandria, VA). All data are expressed as means±SE.
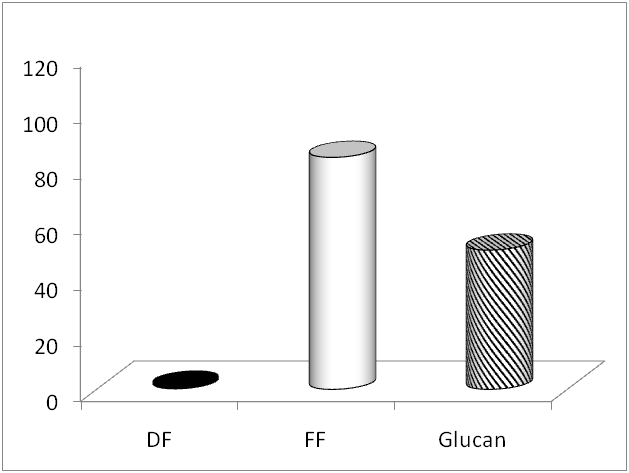
The degree of possible intestinal injury and the NEC incidence were evaluated in all prematurely born rats fed with formula with or without glucan supplementation. The incidence of NEC was strongly decreased from 83.3%(20/24) in the FF group to 50% (10/20) in the FF+Glucan group (Figure 1). Pro-inflammatory and effector cytokines IL-12 and TNF-β belong to the major cytokines associated with NEC. We determined gene expression of these two cytokines in the terminal ileum using RT-PCR technique. We found that the expression of IL-12 did not significantly differ between the groups, whereas expression for TNF-a, was different not only between control and NEC group, but also between NEC and glucan-treated group (Table 1). In addition, we measured the expression of genes coding TLR-2, Tff3, MUC2, iNOS and CXCL1. Results summarized in Table 1 show that expression of TLR-2, iNOS and CXCL1 differed not only between the control and NEC group, but also between the NEC and glucan-treated group, whereas expression of Tff3 and Muc-2 was different only between NEC and glucan-treated animals (Table 1). It is clear that the biggest differences were found in case of iNOS. Numbers of MUC-2-positive cells in the ileum were determined and compared among all three experimental groups. As summarized in Figure 2, experimental NEC induction leads to a strong decrease in the number of MUC2-positive cells, and glucan supplementation did not change this trend. A different situation has been found in the case of Tff3-positive cells. NEC induction led to a strong increase in cells stained for Tff3, but glucan treatment returned these numbers to the control values (Figure 3).
Molecule |
DF |
FF |
FF + Glucan |
TNF-a |
1.58 + 0.28 |
2.41 + 0.52** |
0.58 + 0.06* |
IL-12p35 |
1.0 + 0.40 |
2.03 + 0.52 |
2.29 + 0.52 |
CXCL1 |
1.20 + 0.15 |
2.61 + 0.58** |
1.66 + 0.43* |
iNOS |
1.38 + 0.19 |
35.73 + 8.22** |
2.97 + 0.52* |
TLR-2 |
1.0 + 0.11 |
2.5 + 0.48** |
1.48 + 0.28* |
MUC2 |
1.0 + 0.15 |
0.99 + 0.29 |
2.9 + 0.62* |
Tff3 |
1.0 + 0.10 |
0.94 + 0.14 |
1.75 + 0.23* |
Table 1 Relative mRNA levels.
Mean +/- SEM. *Significant difference between FF and FF + Glucan group, ** significant difference between DF and FF groups at P 0.05 level.
b(1-3)-D-Glucan has been studied for over 50years role and its role as an important immunomodulator has been well established. Multiple studies (currently more than 9,000 scientific publications) have demonstrated that glucans, regardless their solubility, exhibit significant immunostimulating properties, including anti-infectious and anti-tumor activities (for review see.3,22 New studies have shown that glucan exhibits prebiotic properties supporting the growth of probiotic bacteria.23 However, only a few recently published studies evaluated the effects of glucan on the gastrointestinal tract6–7 and none of them used glucan in the model of developing GI tract. In the present study, for the first time, we report that supplementation of glucan into milk formula reduces the incidence and severity of NEC in a neonatal rat model of NEC.
Neonatal NEC is a multifactorial disease with unknown etiology. Decreasing gestational age of newborns strongly correlates with increasing incidence of NEC.24 Composition of infant diet is a critical factor in NEC pathogenesis.25 Dietary glucan was found to regulate levels of inflammatory cytokines and immunoglobulins and subsequently reduce intestinal inflammation.26 Using milk supplemented with glucan opens a novel avenue for use of glucan in prevention of intestinal disorders of premature infants. In our investigation, we used an experimental animal model known to reproduce key risk factors for human NEC, i.e., intestinal immaturity and formula feeding.24 The role of proinflammatory cytokines in NEC pathogenesis is well documented.21,27 IL-12p35 contributes to intestinal inflammation,28 iNos, TNF-a and CXCL1 (rat equivalent of human IL-8) and are all well-established proinflammatory cytokines.29 Our results showed a strong increase in expression of TLR-2, iNOS, CXCL1 and TNF-a (in case of iNOS was the increase 25x) in NEC-induced animals compared to controls. The addition of glucan into the feeding formula reduced the expression of TLR-2, iNOS, CXCL-1 and TNF-a, but iNOS levels were still elevated.
MUC2 is the structural component of the colonic mucus layer and is greatly decreased in inflammatory bowel diseases. The critical role of MUC2 was further supported by findings showing that MUC-2-deficient mice spontaneously developed colitis.30 Bioactive peptide Tff3 is involved in epithelial protection and repair.31 The number of MUC-2-positive cells in ileum was strongly depressed by induction of NEC, and glucan supplementation did not influence this trend. On the other hand, rather different situation was found in case of number of Tff3-positive cells. Significantly higher numbers in animals with NEC were returned to normal values by adding glucan into the formula. These findings corresponded to the results obtained in a study evaluating the effects of pomegranate seed oil.20
In summary, our study demonstrated that orally supplemented glucan offers protection against experimentally induced NEC injury in a neonatal rat model. This effect is probably manifested via reduction of inflammatory response at the site of NEC injury. We believe that the current project might be further developed into a possible novel nutritional factor beneficial to premature neonates.
None.
The author declares no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.