eISSN: 2469-2794


Simple, accurate and reproducible UV spectrophotometric method was established for the assay of Fourosemide based on the formation of Schiff’s bases coupling with aromatic aldehydes such as benzaldehyde, salicylaldehyde, 2-methoxy benzaldehyde, anisaldehyde, vanilline, 2,3,4-chlorobenzaldehyde, 4-nitro benzaldehyde and dimethylaminobenzaldehyde in presence of sulphuric acid, The optical characteristics such as Beer’s law limits, molar absorptivity and Sandell’s sensitivity for the methods are given. Regression analysis using the method of least squares was made to evaluate the slope (b), intercept (a) and correlation coefficient (r) and standard error of estimation (SE) for each aldehyde. Determination of furosemide in bulk form and in pharmaceutical formulations was also incorporated.
Keywords: schiff’s bases; furosemide; benzaldehyde; salicylaldehyde; 2-methoxy benzaldehyde; anisaldehyde; vanilline; 2,3,4-chlorobenzaldehyde
SE, Standard Error of Estimation; Fu, Furosemide; Bz, Benzaldehyde; Sa, Salicylaldehyde; Va, Vanillin; DMAbz, Dimethyl Amino Benzaldehyde
Furosemide (Fu) chemical name is 5-(aminosulfonyl)-4-chloro-2-[(2-furanyl methyl)amino)benzoic acid] (Figure 1).1 It has the following generic names: Frusemide, Fursemide, Aisemide, Beronald, Desdimin, Lasilix and others. The empirical formula is C12H11ClN2O5S corresponds to molecular weight of 330.77. Furosemide is a white to slightly yellow, odourless, almost tasteless crystalline powder, slightly soluble in water, chloroform and ether2 soluble in acetone, methanol, dimethyl formamide1 and in solutions of alkali hydroxides.2 Its melting point is 206°C, the pH of the aqueous solution is in the range 8.9 to 9.3. The UV spectrum of furosemide (0.01mg/ml) in 0.1N NaOH was scanned from 190 to 400 nm using DMS 90 Varian spectrophotometer. It exhibited two maxima at 226 and 272nm. The infrared absorption spectrum for furosemide is shown in Figure 2. Several methods have been reported for the determination of the components of this important drug (furosemide). Titrimetric methods,3‒7 Potentiometric methods8,9 Ultraviolet methods,10‒16 Colorimetric methods.17‒35
The aim of the present study is to develop a simple and accurate method for the selective determination Furosemide in pharmaceutical dosage forms using simple UV technique that can be applied for drug quality control or determination of this active ingredient in different samples.
All chemicals used in this investigation were of the highest purity available. They included Furosemide (Fu), sulphuric acid, and absolute ethanol. The aromatic aldehydes are: benzaldehyde (Bz), salicylaldehyde (Sa), 4-methoxybenzaldehyde(anisaldehyde) (4An), 2-methoxybenzaldehyde (2An), 3-methoxy4-hydroxybenzaldehyde (vanillin) (Va), 2-chlorobenzaldehyde (2Cbz), 3-chlorobenzaldehyde (3Cbz), 4-chlorobenzaldehyde (4Cbz), 4-nitrobenzaldehyde (4Nbz), and dimethylaminobenzaldehyde (DMAbz). Potassium bromide (IR Spectroscopy grade). (Fu) was of analytical or pharmaceutical grades. Octosemide tablets 40mg/tab. (October Pharma for pharmaceutical products, 6th of October city, Egypt. Lasix ampouls 20mg/amp and Lasix tablets 40mg/tab. (Aventis Pharma Company for pharmaceutical products, Morocco). Spectrally chemically pure grade methanol, chloroform, benzene and dichloromethane are purchased from Scharlau, Spain and were used without further purification.
3,1.1 and 2x10-3M Solutions of Fu, were prepared by dissolving the calculated weights 24.8, 9.1 and 16.53 mg respectively in 250mL of methanol.
1x10-2M solutions of Bz, Sa, 4An, Va, 2Cbz, 3Cbz and 4Cbz were prepared by dissolving accurately weighed 265.3, 305.3, 340.4, 380.0, 351.4mg respectively in 250mL volumetric flasks and completing to volume by the desired solvents (Methanol, Chloroform, Benzene and Dichloromethane).
0.5% Ethanolic sulphuric acid v/v solution was prepared by adding 1 mL of concentrated sulphuric acid to 140 mL of absolute ethanol in 200 mL volumetric flask, the solution was mixed well and completed to volume with absolute ethanol.
All spectral and absorbance measurements were carried out at 25ºC by the aid of a SHIMADZUE 160 recording spectrophotometer, using 1cm matched quartz cells. Ftir Shimadzue Model (FTIR 8300) HYPER 1.5 Software version, using KBr Liquid cell.
General procedure
In 100mL beaker, an aliquot of standard Fu solution containing (Fu) as mentioned in Table 1 was transfered, 4mL of the recommended solvents were added, then 5mL of the aromatic aldehyde solution(1x10-2M) was added in the same solvent, the described volume of ethanolic H2SO4 solution 0.5% (v/v) was added and completed to 15mL with the same solvent, heat in a boiling water bath for (13-35) min. for the formation of the corresponding Schiff bases. Cool, transfer quantitatively the residual volume to 5mL measuring flask, complete to the mark with the same solvent and measure the absorbance at the corresponding λmax as mentioned in Table 1.
Solvent |
Bz |
Sa |
4An |
2An |
Va |
2Cbz |
3Cbz |
4Cbz |
|
[Fu]µg/ml |
1 |
20-100 |
10-100 |
May-40 |
20-100 |
2.5-60 |
20-100* |
20-100 |
20-100* |
2 |
|||||||||
3 |
20-100 |
10-100 |
May-40 |
20-100 |
2.5-60 |
20-100 |
20-100 |
20-100 |
|
4 |
|||||||||
Reagent volume ml |
1 |
5 |
5 |
5 |
5 |
5 |
4 |
4 |
4 |
2 |
5 |
5 |
5 |
5 |
5 |
4 |
4 |
4 |
|
3 |
5 |
5 |
5 |
4 |
4 |
5 |
5 |
5 |
|
4 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
|
Acid medium 0.5%v/v (ml) |
1 |
4 |
1 |
3 |
3 |
2 |
4 |
4 |
4 |
2 |
4 |
1 |
3 |
3 |
2 |
2 |
|||
3 |
5 |
1 |
3 |
3 |
3 |
2 |
2 |
2 |
|
4 |
4 |
2 |
3 |
3 |
3 |
2 |
2 |
2 |
|
Boiling tempoC |
|
100 |
|||||||
Heating Time(min.) |
1 |
24 |
24 |
23 |
25 |
20 |
22 |
23 |
23 |
2 |
15 |
15 |
14-Dec |
14-Dec |
13 |
20 |
13 |
20 |
|
3 |
35 |
25 |
23 |
23 |
23 |
25 |
25 |
25 |
|
4 |
10 |
9 |
10 |
10 |
10 |
10 |
9 |
9 |
|
lmn .xam |
1 |
620 |
620 |
620 |
620 |
620 |
705 |
625 |
705 |
2 |
620 |
510 |
640 |
640 |
620 |
705 |
705 |
705 |
|
3 |
625 |
530 |
640 |
640 |
620 |
705 |
705 |
705 |
|
4 |
700 |
640 |
640 |
640 |
620 |
705 |
630 |
630 |
Table 1 Optimum experimental conditions used for the formation of studied furosemide Schiff bases.
1, Methanol; 2, Chloroform; 3, Benzene; 4, Dichloromethane
*In case of 2Cbz and 4Cbz no Formation of Color Occurred using Chloroform.
Preparation of the samples for scanning their IR spectra
Furosemide (Fu) has been scanned as KBr disc as usual in the range 4000cm-1 to 200cm-1. The obtained spectrum was correlated with that reported in Anal Profiles.1 Both Benzaldehyde (Bz) and the formed Schiff bases have been injected in the liquid cell of the IR spectrophotometer and scanned from 4000 cm-1 to 200cm-1 respectively (Figures 3 & 4).
Ground tablets containing 40mg of furosemide (octosemide), (lasix) were ultrasonically dissolved in methanol. The solution was diluted to 200mL with methanol, then filtered; an aliquot of 5mL is diluted to 100mL, so that 1mL of this solution is equivalent to 10µg/mL of Fu. The previous procedure is applied exactly as mentioned in the general procedure.
The available Furosemide ampoules, claimed to contain 20mg Fu, are in the sodium salts water soluble form. For this sake, about 1mL of 1M HCl drop-wisely was added till the complete precipitation of the free base, then the solution was filtered and the precipitate is washed with distilled water (3 times), then the precipitate was dried in a drying oven at 80°C for 15min. After being cooled, transfer the precipitate to 200mL volumetric flask with methanol and complete to the mark. An aliquot of 10mL was diluted to 100 with methanol. 1mL is equivalent to 10µg/mL Fu. Then proceed exactly as mentioned in the general procedure.
The determination of Fu is very important from the point of view of drug analysis. In spite of the availability of different methods for the determination of furosemide which are of higher accuracy and reproducibility, the method of its determination through the formation of Schiff’s bases is very simple, accurate reproducible and needs no sophisticated instruments, only a simple spectrophotometer can perform the determination effectively. A single spectrophotometric method18 for the determination of the drug involving Schiff’s bases formation with single aromatic aldehyde viz. p-dimethyl amino cinnamaldehyde in 65% H2SO4 containing FeCl3 to produce an intensely color measurable at 530nm has been published. Gotardo et al.,31 determined furosemide in pharmaceutical formulations by diffuse reflectance spectroscopy through the formation of the intense violet color of the spot test obtained by the reaction of furosemide with p-dimethyl amino cinnamaldehyde in acid medium at 585nm in the range 7.56 x 10-3 to 6.005 x 10-2molL-1 of the Fu standard solution with a detection limit of 2.49 x 10-3 molL-1. The formed colour was due to the quinonid structure of the imino nitrogen, attached to furanyl methyl radical Equation 1.
While in the present method, the formed color was attributed to the formation of the Schiff’s bases formed from the condensation of the aldehydic groups of the studied aldehydes with the 5-Sulfanoyl anthranilic acid Equation 2.
The IR spectra for Furosemid (Figure 2), benzaldehyde (Figure 3) and the formed Schiff’s base (Figure 4) reveal clearly the formation of Schiff’s bases as proved by the appearance of the azomethine function -C=N at 1654cm-1 reported to give intense absorption in the range from 1690 to 1640cm-1.
Proved also by the weakening or even the disappearance of the amino group –NH2 reported to absorb in the range 3500-3200cm-1. In addition, the Narrow -SO2 group band reported to absorb in the range 1200-1050cm-1, has been appeared as a broad band at 1220cm-1 as a result of being neighboring to the bulky azomethine group with its attached aldehyde. The UV spectrum of the formed Schiff’s bases in the visible range gives high intensity band of deep green color at different wavelengths according to the studied aldehydes (Figures 5 & 6).
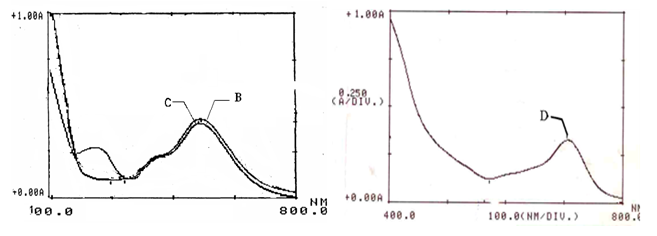
Figure 6 Formation for Schiff’s bases using different Aldehydes with Fu Drug. B, C and D Schiff’s bases with Bz, 4An and 4Cbz, respectively in methanol.
The determination of Fu by formation of the Schiff’s bases method was optimized by investigation of the factors affecting the formation of the colored compounds. These factors were the effect of acidity, effect of the type of the aromatic aldehyde, optimization of the heating time in the water bath, effect of temperature, effect of time on the color development and stability of the formed Schiff’s bases, determination of λmax of the colored product, effect of Fu concentration and obedience to Beer’s law and effect of interfering species. Thus a systematic study of determination of Fu through Schiff’s bases reaction is investigated. Ten aromatic aldehydes have been used in Schiff’s bases formation viz., Bz, Sa, 4An, 2An, Va, 2Cbz, 3Cbz, 4Cbz, 4Nbz. and Dmabz. The effect of sulphuric acid concentration was checked using different volumes (0.1mL-7mL) of sulphuric acid (0.5%v/v) in ethanol, the procedure was conducted as mentioned in the experimental part. The results indicated that 4mL (0.5% v/v) ethanolic H2SO4 was sufficient for achieving maximum absorbance values in the case of Bz, Sa, 4An, 2An, 2Cbz and 4Cbz, while 3mL is found sufficient in the case of Va and 3Cbz. Also the sequences of addition (drug-reagent-acid), (drug-acid-reagent) and (reagent-acid-drug) were tested and all lead to the formation of Schiff’s bases. The best sequence of addition was (drugs-reagent-acid) which gives the highest absorbance.
Effect of the residence time over the steam bath on the reaction
As previously mentioned, the best temperature for the formation Schiff’s bases is boiling water bath (100°C). The optimum time required for the complete formation at this temperature was investigated, as shown in Table 2. The time required for formation of the Schiff’s bases is 23min. for the majority with the used aldehydes except with 2Cbz, 3Cbz and 4Cbz which required 22min. while with Va 21min is sufficient for the formation of the Schiff bases.
Absorbance |
||||||||
Time (min.) |
Bz 620nm |
Sa 620nm |
4An 620nm |
2An 620nm |
Va 620nm |
2Cbz 705nm |
3Cbz 625nm |
4Cbz 705nm |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
10 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
15 |
0 |
0 |
0 |
0 |
0.154 |
0 |
0 |
0 |
19 |
0.118 |
0.172 |
0 |
0 |
0.264 |
0 |
0.124 |
0 |
20 |
0.337 |
0.253 |
0 |
0 |
0.397 |
0.098 |
0.255 |
0.111 |
21 |
0.409 |
0.344 |
0.422 |
0.112 |
0.502 |
0.212 |
0.389 |
0.201 |
22 |
0.489 |
0.42 |
0.52 |
0.269 |
0.502 |
0.346 |
0.419 |
0.356 |
23 |
0.546 |
0.438 |
0.529 |
0.283 |
0.502 |
0.345 |
0.419 |
0.356 |
24 |
0.546 |
0.437 |
0.529 |
0.283 |
0.346 |
0.397 |
0.356 |
|
25 |
0.545 |
0.438 |
0.283 |
0.387 |
||||
Table 2 Effect of time on the formation of furosemide schiff bases.
Effect of time on the stability of the formed color
After complete colour development, the reaction mixture was left to stand for 5 minutes to allow cooling before the measurement, after which the colour remains stable for ≈45min depending on the used aromatic aldehydes.
Effect of solvents
The type of solvent employed affects both the wavelength and intensity of maximum absorption. Table 3 shows the effect of methanol, chloroform, benzene, and dichloromethane, on the intensity of maximum absorption which was very high in the case of using Va as the aromatic aldehyde, and dichloromethane as the organic solvent while, on the contrary, the intensity was very low in the case of benzene and chloroform as solvents.
Solvent |
Methanol |
Chloroform |
Benzene |
Dichloromethane |
|
Bz |
l nm |
620 |
620 |
625 |
700 |
e x14 |
4.6 |
5.8 |
3.7 |
5.3 |
|
Sa |
l nm |
620 |
510 |
530 |
640 |
e x14 |
4.3 |
4.5 |
1.71 |
8.2 |
|
4An |
l nm |
620 |
640 |
640 |
640 |
e x14 |
14 |
1.9 |
3.3 |
3.6 |
|
2An |
L nm |
620 |
640 |
640 |
640 |
e x14 |
3.3 |
1.01 |
3.3 |
3.6 |
|
Va |
L nm |
620 |
620 |
620 |
620 |
e x14 |
12 |
25 |
8.9 |
18 |
|
3Cbz |
l nm |
620 |
640 |
705 |
705 |
e x14 |
5.9 |
5.7 |
1.3 |
3.4 |
|
2Cbz |
l nm |
705 |
- |
705 |
630 |
e x14 |
3.5 |
- |
1.5 |
3.3 |
|
4Cbz |
l nm |
705 |
- |
705 |
630 |
e x14 |
5.2 |
- |
1.5 |
3.3 |
Table 3 Effect of different solvents on absorption.
Selectivity
To demonstrate the selectivity of the proposed methods, the interfering effects of various compounds were examined by the determination 10µg/mL of Fu in the presence of povidone K30 only as excepient where it is soluble in methanol and passes through filtration step. So the interfering of povidone was studied. Its effect on the results does not exceed 0.5% in the case of bz.
Effect of furosemide concentration
Under the optimum conditions, the effect of variation of Fu concentration was investigated. Keeping the aromatic aldehyde concentration constant at 1x10-2M, the absorbance increased as the Fu concentration increased. These results indicate that, the variation of the absorbance with [Fu] is a straight line passing through the origin i.e., obeys Beer’s law up to the concentration of 4.8µg/mL in case of Bz, Sa and 3Cbz at 620, 620 and 625nm, respectively and up to 4µg/mL in the case of 2An, 2Cbz and 4Cbz at 620, 705, and 705nm respectively. While in the case of 4An and Va, [Fu] can be determined only up to 1.6µg/mL and 2.4µg/ml at 620nm. The optical characteristics such as Beer’s law limits, molar absorptivity and Sandell’s sensitivity for the methods are given in Table 4. Regression analysis using the method of least squares was made to evaluate the slope (b), intercept (a) and correlation coefficient (r) and standard error of estimation (SE) for each aldehyde.
Parameters |
Reagents |
|||||||
Bz |
Sa |
4An |
2An |
Va |
2Cbz |
3Cbz |
4Cbz |
|
lmax (nm) |
620 |
620 |
620 |
620 |
620 |
705 |
625 |
705 |
B’L (µg/ml) |
0.9-4.8 |
0.9-4.8 |
0.2-1.6 |
0.8-4.8 |
0.2-2.4 |
1.6-4.8 |
0.9-4.8 |
0.9-4.8 |
Slope (a) |
0.161 |
0.134 |
0.417 |
0.094 |
0.381 |
0.108 |
0.176 |
0.153 |
(e) x106 |
0.487 |
0.403 |
1.26 |
0.285 |
1.152 |
0.326 |
0.532 |
0.463 |
Ss(µg cm-2) |
0.0062 |
0.0075 |
0.0024 |
0.0106 |
0.0026 |
0.0093 |
0.0057 |
0.0065 |
Rb (µg/ml) |
4.01-1.30 |
4.70-1.58 |
2.60-1.46 |
7.19-2.40 |
2.00-1.08 |
6.60-2.35 |
3.58-1.10 |
4.00-1.56 |
Intercept |
0.0011 |
0.0057 |
0 |
0 |
0 |
0 |
0 |
0 |
Cc |
0.99 |
0.998 |
0.995 |
0.989 |
0.998 |
0.998 |
0.999 |
0.995 |
RSD% |
0.533 |
0.521 |
0.82 |
0.769 |
0.592 |
0.762 |
0.722 |
0.411 |
DL (µg/ml) |
0.82 |
0.45 |
0.179 |
0.504 |
0.184 |
1.23 |
0.27 |
0.615 |
Table 4 Analytical data of the determination of furosemide through formation of schiff’s bases using methanol as solvent.
B’L, Beer's Law; (∈), Molar Absorptivity; Ss, Sandell’s Sensitivity; Rb, Ringbom; Cc, Correlation Coefficient, DL, Detection limit
Precision and accuracy of the proposed methods
Five replicates of standard solutions containing 4 different concentrations of Fu were prepared. The overall relative standard deviations were 0.359, 1.016, 1.605, 1.187, 0.686, 1.235, 0.614 and 1.109 for the formed Schiff’s bases with Bz, Sa, 4An, 2An, Va, 2Cbz, 3Cbz and 3Cbz reagents respectively. The results are given in Table 5.
Reagents |
[Fu] (µg/5ml) |
% Recovery |
||
Taken |
Mean Found±SD |
RSD% |
||
8 |
8.0025 ± 0.041 |
0.512 |
100.03 |
|
Benzaldehyde |
12 |
12.035 ± 0.059 |
0.49 |
100.3 |
16 |
16.095 ± 0.052 |
0.323 |
100.5 |
|
20 |
20.137 ± 0.023 |
0.114 |
100.6 |
|
Mean |
0.359 |
|||
Salicylaldehyde |
8 |
7.975 ± 0.037 |
0.464 |
99.68 |
12 |
12.035 ± 0.057 |
0.474 |
100.3 |
|
16 |
15.88 ± 0.312 |
1.965 |
99.2 |
|
20 |
20.04 ± 0.233 |
1.162 |
99.84 |
|
Mean |
1.016 |
|||
4-Methoxy Benzaldehyde |
2 |
2.005 ± 0.033 |
1.64 |
100.25 |
4 |
3.982 ± 0.063 |
1.58 |
99.5 |
|
6 |
6.007 ± 0.114 |
1.89 |
100.12 |
|
8 |
8.0725 ± 0.106 |
1.31 |
100.9 |
|
Mean |
1.605 |
|||
2-Methoxy Benzaldehyde |
4 |
3.977 ± 0.052 |
1.31 |
99.43 |
8 |
7.92 ± 0.155 |
1.95 |
98.96 |
|
12 |
12.077 ± 0.066 |
0.546 |
100.46 |
|
16 |
15.88 ± 0.150 |
0.944 |
99.26 |
|
Mean |
1.187 |
|||
Vanilline |
2 |
2.007 ± 0.025 |
1.245 |
100.3 |
4 |
4.000 ± 0.016 |
0.4 |
100 |
|
6 |
6.02 ± 0.046 |
0.764 |
100.33 |
|
8 |
8.0225 ± 0.027 |
0.336 |
100.28 |
|
Mean |
0.686 |
|||
2-Chlorobenzaldehyde |
8 |
8.041 ± 0.135 |
1.67 |
98.96 |
12 |
12.087 ± 0.065 |
0.537 |
100.64 |
|
16 |
16.26 ± 0.307 |
1.888 |
99.26 |
|
20 |
19.977 ± 0.169 |
0.845 |
99.88 |
|
Mean |
1.235 |
|||
3-Chlorobenzaldehyde |
8 |
7.972 ± 0.082 |
1.028 |
99.62 |
12 |
12.04 ± 0.100 |
0.83 |
100.33 |
|
16 |
15.94 ± 0.019 |
0.119 |
99.26 |
|
20 |
20.025 ± 0.096 |
0.479 |
100.12 |
|
Mean |
0.614 |
|||
4-Chlorobenzaldehyde |
8 |
7.95 ± 0.063 |
0.792 |
99.37 |
12 |
11.98 ± 0.068 |
0.567 |
99.83 |
|
16 |
16.16 ± 0.235 |
1.454 |
100.81 |
|
20 |
20.005 ± 0.325 |
1.624 |
100.02 |
|
Mean |
1.109 |
|||
Table 5 Tests of recovery of the method on samples of pure drug.
Analytical applications
Octosemide tables and lasix ampoules: The absorbance of the formed Schiff’s bases was measured at λmax corresponding to the used aromatic aldehydes as illustrated in Table 1. The concentration was calculated from the previously constructed calibration curves. Average recovery was 98.2% and RSD % range (0.6-1.3). The absorbance of the formed Schiff’s bases was measured at λmax 620nm and was referred to pre constructed calibration curves. Average recoveries were 100.9 and RSD% range (1.54-1.98). Table 6 represents the obtained data using benzaldehyde reagent.
[Fu]mg/5ml |
RSD % |
Recovery % |
||
Dosage form |
Taken |
Mean found±SD |
|
|
Octosemide tab. |
10 |
9.28±0.124 |
1.325 |
92.8 |
15 |
14.53±0.153 |
1.053 |
96.87 |
|
20 |
20.99±0.126 |
0.6 |
104.95 |
|
Mean |
0.993 |
98.206 |
||
Lasix tab. |
10 |
10.21±0.219 |
2.145 |
102.1 |
15 |
16.06±0.033 |
0.205 |
107.07 |
|
20 |
19.99±0.232 |
1.161 |
99.95 |
|
Mean |
1.17 |
103.039 |
||
Lasix amp. |
10 |
9.17±0.182 |
1.985 |
91.7 |
15 |
16.28±0.287 |
1.763 |
108.53 |
|
20 |
21.12±0.326 |
1.544 |
105.6 |
|
Mean |
1.779 |
100.944 |
||
Table 6 Tests of precision of the method on pharmaceutical preparations.
None.
The author declares that there are no conflicts of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.