eISSN: 2473-0815


Diabetes mellitus is a chronic illness that requires continuing medical care and ongoing patient self-management education and support to prevent acute complications and to reduce the long-term complications. In certain populations such as Asians, particularly Indians the prevalence of diabetes is high. Obesity and advanced gestational age are the other risk factors for gestational diabetes. Perinatal mortality and morbidity are high in untreated diabetic pregnancies. However, this has decreased considerably with screening, antepartum fetal surveillance and insulin therapy. This article deals about the definition, risk factors, pathogenesis, screening and management of diabetes in pregnancy and its complications on mother and fetus and its long-term adverse effects and prevention.
Keywords: diabetes mellitus, gestational diabetes, screening, fetal surveillance, insulin therapy, hyprothyroidism
Definition
THE AMERICAN DIABETES ASSOCIATION defines GESTATINAL DIABETES as ‘any degree of glucose intolerance with onset or first recognition during pregnancy ‘where as pregestational diabetes comprises of type 1 and type 2 diabetes occurring prior to pregnancy.1
History
Before the discovery of insulin, and in the case of uncontrolled diabetes, infertility was the rule. Many of the cases tend to have amenorrhoea and in the pre-insulin era only 2 percent of diabetic patients conceived. Increasing number of diabetics is now becoming pregnant and the fertility rate has increased to 25 to 30 percent with the discovery of insulin. Insulin therapy was introduced nearly 100 years ago and remains perhaps the most important landmark in the care of pregnancy for the diabetic woman. Before insulin became available, pregnancy was not advised because it was likely to be accompanied by fetal mortality and also came with a substantial risk for maternal death. Over the past 30 years, however, management techniques have been developed that can prevent many complications associated with the diabetic pregnancy. These advances, based on our understanding of the pathophysiology, have resulted in perinatal mortality rates in optimally managed cases that approach those of the normal population. This dramatic improvement in perinatal outcome can be largely attributed to clinical efforts to establish improved maternal glycemic control both before conception and during gestation.2
Epidemiology
Diabetes is a major public health problem in India. The prevalence of diabetes ranges from 4.6% to14% in urban areas and 1.7% to 13.5% in rural areas. IN India it is estimated that 62 million people are suffering from type 2 DM which is expected to increase to 79.4 million by 2025 and homes second largest number globally.IN INDIA, it is estimated that about 4 million are affected by GDM and across India, the prevalence of GDM ranges from 3.8% to 41%. In 2017, members of the International Diabetes Federation decided a theme for the year “women and Diabetes” especially for those pregnant and how their new born may be affected by the condition.3
Class |
Diabetes onset age (yr) |
Duration (yr) |
Vascular Disease |
Need for insulin or oral agent |
Gestational diabetes |
||||
A1 |
Any |
Any |
- |
- |
A2 |
Any |
Any |
- |
+ |
Pregestational diabetes |
||||
B |
>20 |
<10 |
- |
+ |
C |
10 to 19 |
or 10 to 19 |
- |
+ |
D |
<10 |
or>20 |
+ |
+ |
F |
Any |
Any |
+ |
+ |
R |
Any |
Any |
+ |
+ |
T |
Any |
Any |
+ |
+ |
H |
Any |
Any |
+ |
+ |
Table 1 Modified White Classification Of Pregnant Diabetic Women
Nod om White Prynany complaindts dn] Med 147409
Carbohydrare metabolism in pregnancy
In humans, normal glucose tolerance is maintained because of a balance between adequate insulin secretion and insulin sensitivity. The secretory response of the pancreatic b-cells to glucose (particularly in the early phase) and the sensitivity of the glucose utilizing tissues to insulin determine the ability of insulin to dispose of Carbohydrates.4 Pregnancy alters carbohydrate metabolism but adaptation occurs and there is no effect on mother and foetus as insulin secretion increases. When there is abnormal response there is increased foetal risk. Abnormal glucose tolerance occurs when the pancreatic b-cells output does not meet tissues insulin needs in response to changes in insulin resistance.4,5
Pregnancy is a diabetogenic, hyperlipidaemic ang glucosuric state. After mid pregnancy resistance to insulin develops due to placental reduction of anti-insulin hormones like human placenta lactogen, cortisol, oestrogen and progesterone.
The effect of these hormones causes:6
Normally glucose levels stay constant between 4to 4.4 mmol/l except after meals. With increasing insulin resistance homeostasis can only be maintained by doubling the insulin secretion from the end of 1st to 3rd trimester.
Fasting blood glucose decreases at early pregnancy and continuously during gestation.7 Insulin sensitivity declines with advancing gestation to reach at late gestation (34–36 weeks) 50–60% of pre-gravid state.8
As a reflection of insulin resistance occurrence, fasting insulin concentrations increase. The changes in insulin sensitivity are inversely related to changes in maternal body fat mass.9 Hepatic glucose production increases during pregnancy suggest that the defect in insulin action also targets the liver. Catalano et al.,10,11 found a significant increase in basal endogenous glucose production at the end of gestation in spite of the important increase in fasting insulin concentration.
First half of pregnancy (Anabolic)
Second half of pregnancy (Catabolic)
Glycosuria in pregnancy
It is an early indicator of gestational diabetes. During pregnancy renal threshold is diminished due to combined effect of increases GFR and impaired tubular reabsorption of glucose, which is most common in midpregnancy. Repeat and random urine sample taken on one or more occasion throughout pregnancy reveal glycosuria in 5 to 50% cases. Gylcosuira on one occasion before 20 weeks is an indicator for glucose tolerance test.
Inflammatory mediators and their role in pregnancy and diabetes
Pregnancy has been characterized as a chronic low-grade inflammatory condition because of the increase in activation of circulating blood leukocytes.9 the inflammation of pregnancy is further enhanced by maternal prepregnancy obesity. This increase in low-grade inflammation, particularly observed in obese women, has been related to increases in macrophage infiltration in both maternal white adipose tissue and the placenta. The increase in inflammation has been associated with increased circulating C-reactive protein (CRP) and interleukin-6 (IL-6). Both of these factors may exacerbate the increased insulin resistance previously noted in obese women with normal glucose tolerance because of effects on the postreceptor insulin-signaling cascade. These inflammatory cytokines may then relate to increased substrate availability for the developing fetus and resultant macrosomia. TNF-α was the strongest independent predictor of insulin sensitivity in pregnancy, accounting for about half of the variance in the decrease in insulin sensitivity during gestation.12
Glucose transport
Placental glucose transport is a process that takes place through facilitated diffusion. Glucose transport is dependent on a family of glucose transporters referred to as the GLUT glucose transporter family. The principal glucose transporter in the placenta is GLUT1, which is located in the syncytiotrophoblast. GLUT1 is located on both the microvillus and basal membranes. Basal membrane GLUT1 may be the rate limiting step in placental glucose transport. A twofold to threefold increase is seen in the expression of syncytiotrophoblast glucose transporters with advancing gestation. Although GLUT3 and GLUT4 expression have been identified in placental endothelial cells and intervillous nontrophoblastic cells, respectively, the role they may play in placental glucose transport remains speculative.
Pederson hypothesis
IN 1920 Jorgen PEDERSON stated that maternal hyperglycemia results in fetal hyperglycemia and hence it results in hypertrophy of fetal pancreatic islet cells resulting in insulin hypersecretion.
Role of adipoinsular axis
An endocrine feedback loop called ad adipoinsular axis connects the endocrine pancreas with adipose tissue and the brain. This axis regulates hunger and fat storage through the hormones named insulin and leptin. Insulin is responsible for promoting the development of fat mass and leptin production. Leptin reduces energy intake and supresses the insulin secretion via leptin receptors on pancreatic beta cells. Abnormal functioning of this axis may lead to hyperphagia, dysregulation of the energy balance and excessive adiposity.
Growth hormone in diabetes
Growth hormone may antagonise the peripheral effect of insulin, and may cause it to be utilised in the tissues at such a high rate, that the level of insulin in the blood falls and as a result the islet may be stimulated even more to produce insulin.
Vitamin D3
The prevalence of type 1 diabetes has inversely corelated. Low vitamin D synthesis may be important in the pathogenesis of type 1 diabetes. Lack od vitamin D3 supplementation in infants has been associated with increased risk of type 1 diabetes later in life.
Anaemia with diabetes
Occurs due to following reasons
Thyroid disorders and diabetes in pregnancy
Thyroxine is an anti-insulin hormone. Prevalence of 13.4% of thyroid diseases in diabetes with the highest prevalence in type 1 female diabetics (31.4%) and lowest in type 2 male diabetics (6.9%). Thyroid hormones raise the rate of hepatic glucose output, increases gluconeogenesis, increases availability of substrates like amino acids and free fatty acids. Thus, hyperthyroidism causes hyperglycaemia and worsens the pre-existing impaired glucose tolerance.
ADA recommends testing individual with type 1 diabetes for antithyroid peroxide antibodies soon after diagnosis.
Thyroid hormones increase basal metabolic rate by
Screening
Gestational diabetes is asymptomatic and hence the need for screening is essential. Two methods of screening are in practice today
All pregnant women are screened in the universal screening protocol while selective screening is done only in the presence of risk factors for gestational diabetes.
Criteria of the fifth international workshop-conference on gestational diabetes
GDM risk assessment: should be ascertained at the first prenatal visit.
Low risk:
Blood glucose testing not routinely required if all the following are present:
Average risk
Perform blood glucose testing at 24 to 28 weeks using either: Two-step procedure: 50-g oral glucose challenge test (GCT), followed by a diagnostic 100-g OGTT for those meeting the threshold value in the GCT
One-step procedure: diagnostic 100-g OGTT performed on all subjects.
High risk
Perform blood glucose testing as soon as feasible, using the procedures described above, if one or more of these are present:
Screening should be performed between 24 and 28 weeks’ gestation in those women not known to have glucose intolerance earlier in pregnancy. This 50-g screening test is followed by a diagnostic 100-g, 3-hour oral glucose tolerance test (OGTT) if screening results meet or exceed a predetermined plasma glucose concentration.
Diabetes in pregnancy study group in india (DIPSI)
DIPSI has recommended single step procedure for diagnosing GDM In community. DIPSI diagnostic criteria of 2hr post prandial level of ≥140mg/dl which is similar to WH0 criteria. This was developed due to practical difficulty in performing glucose tolerance in fasting sate, challenge of women revisiting the antenatal clinic and that too in fasting state.14,15
The hyperglycemia and adverse pregnancy outcome study
This was a 7-year international epidemiological study of 23,325 pregnant women at 15 centres in nine countries (HAPO Study Cooperative Research Group, 2008). The investigation analysed the association of various levels of glucose intolerance during the third trimester with adverse infant outcomes in women with gestational diabetes. Between 24 and 32 weeks’ gestation, the general population of pregnant women underwent a 75-g OGTT after an overnight fasting. Blood glucose levels were measured fasting and then 1 and 2 hours after glucose ingestion. These values were then correlated with rates for birthweight >90th percentile (LGA), primary caesarean delivery, neonatal hypoglycaemia, and cord-serum C-peptide levels >90th percentile. Odds of each outcome were calculated using the lowest category for example, fasting plasma glucose ≤75 mg/dL—as the referent group. Their findings in general supported the supposition that increasing plasma glucose levels were associated with increasing adverse outcomes.16
International association of diabetes and pregnancy study group
The IADPSG sponsored a workshop conference on the diagnosis and classification of gestational diabetes in 2008. After reviewing the results of the HAPO study, a panel developed recommendations for the diagnosis and classification of hyperglycemia during pregnancy. This panel allowed for the diagnosis of overt diabetes during pregnancy as shown .It also recommended a singlestep approach to the diagnosis of gestational diabetes using the 75-g, 2-hour OGTT. Thresholds for fasting, 1-, and 2-hour values based on mean glucose concentrations from the entire HAPO study cohort were considered. These glucose level thresholds were derived using an arbitrary 1.75 odds ratio of outcomes such as LGA birthweight and cord serum C-peptide levels >90th percentile. Only one of these thresholds would need to be met or exceeded to make the diagnosis of gestational diabetes. Feldman and co-workers (2016) evaluated the implementation of the IADPSG paradigm in a before-after analysis that included more than 6000 women. The new strategy was associated with a significant increase in gestational diabetes diagnosis rates but not with reduced macrosomia rates compared with a two-step approach. Remarkably, they identified a higher primary caesarean delivery rate associated with adoption ofthe IADPSG recommendations. The ADA (2013, 2017a) initially recommended adopting this new approach, however, based on benefits inferred from trials in women identified using a two-step approach described, they now concede that data support a two-step strategy as well (Figure 1).16
Controversies regarding best screening method
The IADPSG and ADA recognize that using the one-step approach would likely increase the number of women with a GDM diagnosis because only one abnormal value is needed for diagnosis. Although this may lead to increased health care costs, the ADA believes that the benefits outweigh these disadvantages.17 Data are unavailable from randomized controlled trials regarding outcomes for these additional women who’s GDM would be diagnosed by the one-step method. In the two-step approach, two different sets of glucose thresholds exist: Carpenter-Coustan and National Diabetes.
Data Group (NDDG). The Carpenter-Coustan thresholds are lower than the NDDG thresholds, resulting in higher rates of GDM diagnoses. Uses of the Carpenter-Coustan criteria increases diagnosis by 30%–50%.18 Comparative trials are limited, making it difficult to recommend one criterion over another. One recent study compared the two diagnostic criteria and their effects on outcomes. This was a secondary analysis of a trial involving the treatment of mild GDM in 958 patients. Results showed that treatment with nutritional counselling, dietary therapy, and, in some cases, insulin provided similar reductions in the incidence of pregnancy-induced hypertension, shoulder dystocia, caesarean delivery, and macrosomia, regardless of which two-step diagnostic criteria were used. Clinicians and institutions should select one criteria to use consistently, with local rates of diabetes and availability of resources for managing GDM factored into that decision. A recent systematic review analysed 38 studies to assess different diagnostic thresholds for GDM on maternal and fetal outcomes in the absence of treatment for GDM. The results showed that women with GDM, regardless of the diagnostic criteria used, consistently had higher rates of caesarean section, shoulder dystocia, and large for gestational age (LGA) infants. Macrosomia was significantly higher with the twostep approach, but not the one-step approach. The authors concluded that higher glucose thresholds did not consistently have higher maternal or fetal risks, and further research is needed to determine which diagnostic criteria are associated with the best outcomes.17,18
Diabetic screening in a non-pregnant state
Fetal effects
Spontaneous abortion: Early miscarriage is associated with poor glycemic control. Up to 25 percent of diabetic gravidas have an early pregnancy loss. Those whose HbA1c concentrations were >12 percent or whose preprandial glucose concentrations were persistently >120 mg/dL had an elevated risk.
Malformations
Malformations: The incidence of major malformations in women with type 1 diabetes is at least doubled and approximates 11 percent. Cardiovascular malformations accounted for more than half of the anomalies. The risk of an isolated cardiac defect was fivefold higher in women with type 1 diabetes. The caudal regression sequence is a rare malformation frequently associated with maternal diabetes.
Three interrelated molecular chain reactions have been linked to congenital malformations. They are
Organ system |
Type 1 DM |
Type 2 DM |
DM |
n=482 |
n=4166 |
n=31,700 |
|
Total |
55 |
454 |
22203 |
Cardiac |
38 |
272 |
1129 |
Muscaloskeletal |
1 |
31 |
231 |
Urinary |
3 |
28 |
260 |
CNS |
1 |
13 |
64 |
GI |
1 |
30 |
164 |
Other |
11 |
80 |
355 |
Table 2 Major Congenital Anomalies in 36,345 Neonates Born to Women with Diabetes between 2004 and 2011
Abbreviations: CNS, central nervous system; DM, diabetes mellitus; GDM, gestational diabetes; GI, gastrointestinal
The frequency of major congenital malformations in newborns of women with pregestational diabetes stratified by hemoglobin A1c levels at first prenatal visit. Most common malformations are in cardiovascular system. It includes transposition of great vessels, ventricular and atrial septal defects, hypoplasia of left ventricle, situs inversus, aortic anomalies and complex cardiac anomalies. The ratio of cardiac anomalies is fivefold higher in diabetic. Caudal agenesis/ caudal regression are highly suggestive of diabetic fetopathy. It is a rare malformation but diagnosed upto 400 times more frequently in diabetic pregnancies and is nearly pathognomonic. There is 10 fold increases in the incidence of central nervous system malformations in infants of diabetic mother including anencephaly, holoprosencephaly, open spina bifida, microcephaly, encephalocele, and meningomyelocele. Gastrointestinal system malformations including trachea oesophageal fistula, bowel atresia, imperforate anus is also increased in diabetic gestation Genitourinary system anomalies including absent kidneys (leading to potter syndrome) polycystic kidneys, double ureter are also common in diabetes complicating pregnancy (Table 3).
Anomaly |
Ratio of incidence |
Caudal regression |
252 |
Situs inversus |
84 |
Spina bifida, hydrocephaly, CNS defects |
2 |
Anencephaly |
3 |
Cardiac anomaly |
4 |
Anal/rectal agenesis |
3 |
Renal anomalies |
5 |
Agenesis |
4 |
Cystic kidney |
4 |
Duplex ureter |
23 |
Table 3 Ratio of Incidence of Congenital Anomalies in Comparison with General Population
Unexplained stillbirths are associated with poor glycemic control. Fetuses of diabetic mothers often have elevated lactic acid levels. Mean umbilical venous blood pH was lower in diabetic pregnancies and was significantly related to fetal insulin levels. Such findings support the hypothesis that hyperglycemia-mediated chronic aberrations in oxygen and fetal metabolite transport may underlie these unexplained fetal deaths. Aside from hyperglycemia alone, maternal ketoacidosis can cause fetal death. Explicable stillbirths due to placental insufficiency also occur with increased frequency in women with overt diabetes, usually in association with severe preeclampsia. In the prior California study of nearly a half million singleton deliveries, the fetal death risk was sevenfold higher in women with hypertension and pregestational diabetes compared with the threefold increased risk associated with diabetes alone. Stillbirth rates are also greater in women with advanced diabetes and vascular complications.
Macrosomia
Macrosomia is defined as an estimated fetal weight of more than 4000gms to 4500gms or more than 90th percentile at any gestational age. It occurs in 25% to 42% in hyperglycemic pregnancies. Diabetic macrosomia is characterised by specifically by a large fetal abdominal circumference and decreased head to abdominal circumference ratio because fetal hyperinsulinemia leads to abdominal fat distribution.
Altered fetal growth
Diminished growth may result from congenital malformations or from substrate deprivation due to advanced maternal vascular disease. That said, fetal overgrowth is more typical of pregestational diabetes. Maternal hyperglycemia prompts fetal hyperinsulinemia, and this in turn stimulates excessive somatic growth. Except for the brain, most fetal organs are affected by the macrosomia. Diabetic woman have excessive fat deposition on the shoulders and trunk, which predisposes to shoulder dystocia or caesarean delivery (Figure 2).
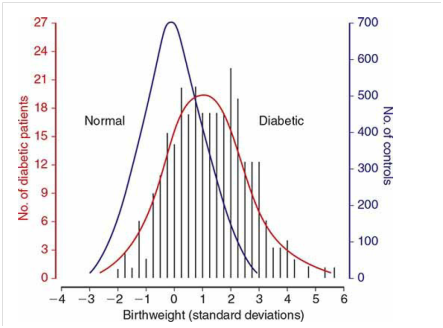
Figure 2 Distribution of birthweight standard deviations from the normal mean for gestational age in 280 newborns of diabetic mothers and in 3959 neonates of nondiabetic mothers.
Neonatal effects
Modern neonatal care has reduced neonatal death rates due to immaturity; however neonatal morbiditydue to preterm birth continues to be a serious consequence of pregestational diabetes. Preterm neonates, those born to diabetic women treated with insulin prior to pregnancy were at greater risk for necrotizing enterocolitis and late-onset sepsis than neonates of mothers without diabetes.
Respiratory distress syndrome: Gestational age rather than overt diabetes is likely the most significant factor associated with respiratory distress syndrome. Indeed, very-low-birth weight neonates delivered between 24 and 33 weeks’ gestation, rates of respiratory distress syndrome in newborns of diabetic mothers were not higher compared with rates in neonates of nondiabetic mothers.
Hypoglycemia
Newborns of a diabetic mother experience a rapid drop in plasma glucose concentration after delivery. This is attributed to hyperplasia of the fetal β-islet cells induced by chronic maternal hyperglycemia. Low glucose concentrations—defined as <45 mg/dL—are particularly common in newborns of women with unstable glucose concentrations during labor.
Hypocalcemia
Defined as a total serum calcium concentration <8 mg/dL in term newborns, early onset hypocalcemia is one of the potential metabolic derangements in neonates of diabetic mothers. Its cause has not been explained. Theories include aberrations in magnesium–calcium economy, asphyxia, and preterm birth.
Hyperbilirubinemia and polycythemia
The pathogenesis of hyperbilirubinemia in neonates of diabetic mothers is uncertain. A major contributing factor is newborn polycythemia, which raises the bilirubin load. Polycythemia is thought to be a fetal response to relative hypoxia. According to Hay (2012), the sources of this fetal hypoxia are hyperglycemia-mediated elevations in maternal affinity for oxygen and fetal oxygen consumption. Together with insulin-like growth factors, this hypoxia leads to elevated fetal erythropoietin levels and red cell production. Fetal renal vein thrombosis is reported to result from polycythemia.
Cardiomyopathy
Newborns of diabetic pregnancies may have hypertrophic cardiomyopathy that primarily affects the interventricular septum. In the first trimester, fetal diastolic dysfunction was already evident. In the third trimester, the fetal interventricular septum and right ventricular wall were thicker in fetuses of diabetic mothers. Most affected newborns are asymptomatic following birth, and hypertrophy usually resolves in the months after delivery.
Long-term cognitive development
Intrauterine metabolic conditions have long been linked to neurodevelopment in offspring. The intelligence quotient of those whose mothers had diabetes during pregnancy averaged 1 to 2 points lower. DeBoer and associates (2005) demonstrated impaired memory performance in infants of diabetic mothers at age 1 year. Results from the Childhood Autism Risks from Genetics and the Environment (CHARGE) study indicated that autism spectrum disorders or developmental delay were also more common in children of diabetic women.
Inheritance of diabetes
The risk of developing type 1 diabetes if either parent is affected is 3 to 5 percent. Type 2 diabetes has a much stronger genetic component. If both parents have type 2 diabetes, the risk of developing it approaches 40 Percent.
Acute complications
Infections: The rates of many infections are higher in diabetic pregnancies. Common ones include candidal vulvovaginitis, urinary and respiratory tract infections, and puerperal pelvic sepsis.
Hydramnios
Polyhydramnios occurs in 3% to 30% of diabetic pregnancies, 30 times the rate of non-diabetic gestation. Diabetes alone is the leading cause of polyhydramnios. Mechanism of polyhydramnios includes fetal glycemic load resulting in polyuria, decreased fetal swallowing and GI obstructions, presence of sugar within amniotic fluid may irritate the amnion to produce increased amounts of liquor.
Preterm delivery
Overt diabetes is an undisputed risk factor for preterm birth. Almost 60 percent were indicated preterm births, that is, due to obstetrical or medical complications.
Severe hypoglycemia
Severe hypoglycaemia requiring hospitalization occurs in 45% of mothers with type 1 DM. Vomiting in early pregnancy also predisposes to diabetes to low blood sugars. Symptoms include nausea, vomiting, headache, diaphoresis, tremors, blurred vision, weakness, hunger, confusion, paresthesia, and stupor. Dignosis is measuring blood sugar of less than 60mg/dl.
Treatment starts with 4 oz of juice or glucose. Assess serum glucose after 15 to 20 minutes and repeat feeding until blood sugar is more than 70mg/dl followed by complex carbohydrate or a scheduled meal or a snack. If patient is unable to tolerate oral fees administer subcutaneous injection of glucagon or an ampule of 10% dextrose bolus followed by IV fluids containing 5% dextrose.
THE SOMOGYI EFFECT is rebound hyperglycemia after hypoglycaemia secondary to counter regulatory hormone release.it occurs in the middle of the night but can happen after any hypoglycemic attacts, manifesting a wide variations in blood glucose levels over a short period of time(between 2AM to 6AM). Diagnosis is by checking additional blood glucose at 3AM to identify unrecognised hypoglycmia. Treatment involves adding or modifying a night time snack or decreasing overnight insulin dose in order to better match insulin needs with diatery intake.
THE DAWN PHENOMENON is also an early morning hyperglycemia possibly due to normal night production of growth hormone, catecholamines and cortisol. It is also diagnosed by checking blood glucose at 3AM. Treatment includes increasing the night dose of insulin to cover the effect of normal hormones.
Type 1 diabetes was at increased risk for hypertension and respiratory complications compared with nondiabetic women. And, 10,126 mothers with type 2 diabetes had an elevated risk for depression, hypertension, infection, and cardiac or respiratory complications compared with pregnant controls.
Diabetic ketoacidosis
This serious complication develops in approximately 1 percent of diabetic pregnancies and is most often encountered in women with type 1 diabetes. It is increasingly being reported in women with type 2 or even those with gestational diabetes. Diabetic ketoacidosis (DKA) may develop with hyperemesis gravidarum, infection, insulin noncompliance, β-mimetic drugs given for tocolysis, and corticosteroids given to induce fetal lung maturation. DKA results from an insulin deficiency combined with an excess in counter-regulatory hormones such as glucagon. This leads to gluconeogenesis and ketone body formation. The ketone body β-hydroxybutyrate is synthesized at a much greater rate than acetoacetate, which is preferentially detected by commonly used ketosis-detection methods. Therefore, serum or plasma assays for β-hydroxybutyrate more accurately reflect true ketone body levels. Of gravidas with DKA, fewer than 1 percent die, but perinatal mortality rates from a single episode of DKA may reach 35 percent. Diabetic ketoacidosis (DKA) may develop with hyperemesis gravidarum, infection, insulin noncompliance, β-mimetic drugs given for tocolysis, and corticosteroids given to induce fetal lung maturation. DKA results from an insulin deficiency combined with an excess in counter-regulatory hormones such as glucagon. This leads to gluconeogenesis and ketone body formation. The ketone body β-hydroxybutyrate is synthesized at a much greater rate than acetoacetate, which is preferentially detected by commonly used ketosis-detection methods. Therefore, serum or plasma assays for β-hydroxybutyrate more accurately reflect true ketone body levels.
Of gravidas with DKA, less than 1 percent die, but perinatal mortality rates from a single episode of DKA may reach 35 percent. The mean glucose level for pregnant women with DKA was 380 mg/dL, and the mean HbA1C value was 10 percent.
Laboratory assessment
Obtain arterial blood gases to document degree of acidosis present; measure glucose, ketones, and electrolyte levels at 1- to 2-hour intervals
Insulin
Low-dose, intravenous
Loading dose: 0.2–0.4 U/kg
Maintenance: 2–10 U/hr
Fluids
Isotonic sodium chloride
Total replacement in first 12 hours of 4–6 L
1 L in first hour
500–1000 mL/hr for 2–4 hours
250 mL/hr until 80 percent replaced
Glucose
Begin 5-percent dextrose in normal saline when glucose plasma level reaches 250mg/dL (14 mmol/L)
Potassium
If initially normal or reduced, an infusion rate up to 15–20 mEq/hr may be required; if elevated, wait until levels decrease into the normal range, then add to intravenous solution in a concentration of 20–30 mEq/L
Bicarbonate
Add one ampule (44 mEq) to 1 L of 0.45 normal saline if pH is <7.1
Acute vascular complications
Women with diabetes presenting with acute coronary syndrome or acute stroke have bad prognosis mainly due to increased platelet reactivity and more severe cardiovascular disease.
Preeclampsia
Pregnancy-associated hypertension is the complication that most often forces preterm delivery in diabetic women. The incidence of chronic and gestational hypertension especially preeclampsia is remarkably increased. Preeclampsia developed three to four times more often in women with overt diabetes. Moreover, those diabetics with coexistent chronic hypertension were almost 12 times more likely to develop preeclampsia. Women with type 1 diabetes in more advanced White classes of overt diabetes, who typically exhibit vascular complications and have preexisting nephropathy, are more likely to develop preeclampsia. This rising risk with duration of diabetes may be related to oxidative stress, which plays a key role in the pathogenesis of diabetic complications and preeclampsia.
Chronic complictions
Diabetic nephropathy
Approximately 5 percent of pregnant women with diabetes already have renal involvement. Approximately 40 percent of these will develop preeclampsia. In those with microproteinuria, this incidence may not be as high. The rates of preterm delivery, birthweight <2500 g, and growth restriction were significantly higher in neonates of diabetic women with microproteinuria compared with those of diabetic gravidas without proteinuria. Clinically detectable nephropathy begins with microalbuminuria—30 to 300 mg/24 hours. This may manifest as early as 5 years after diabetes onset. Macroalbuminuria—more than 300 mg/24 hours—develops in patients destined to have end-stage renal disease. Hypertension almost invariably develops during this period, and renal failure ensues typically in the next 5 to 10 years. The incidence of overt proteinuria is nearly 30 percent in individuals with type 1 diabetes and ranges from 4 to 20 percent in those with type 2 diabetes. Regression is common and, presumably from improved glucose control, the incidence of nephropathy with type 1 diabetes has declined.
Diabetic retinopathy
Retinal vasculopathy is a highly specific complication of both type 1 and type 2 diabetes. The first and most common visible lesions are small microaneurysms followed by blot hemorrhages that form when erythrocytes escape from the aneurysms. These areas leak serous fluid that creates hard exudates. Such features are termed background or nonproliferative retinopathy. With increasingly severe retinopathy, the abnormal vessels of background eye disease become occluded, leading to retinal ischemia and infarctions that appear as cotton wool exudates. These are considered preproliferative retinopathy. In response to ischemia, neovascularization begins on the retinal surface and out into the vitreous cavity. Vision is obscured when these vessels bleed. Laser photocoagulation before hemorrhage reduces the rate of visual loss progression and blindness by half. The procedure may be performed during pregnancy when indicated. Other risk factors that have been associated with progression of retinopathy include hypertension, higher levels of insulin-like growth factor-1, placental growth factor, and macular edema identified in early pregnancy. “Acute” rigorous metabolic control during pregnancy has been linked to acute worsening of retinopathy.
Diabetic neuropathy
Peripheral symmetrical sensorimotor diabetic neuropathy is uncommon in pregnant women. But, a form of this, known as diabetic gastropathy, can be troublesome during pregnancy. It causes nausea and vomiting, nutritional problems, and difficulty with glucose control. Women with gastroparesis are advised that this complication is associated with a high risk of morbidity and poor perinatal outcome
Maternal |
Fetal |
Preeclampsia |
Macrosomia |
Pyelonephritis |
Sudden IUD |
polyhydramnios |
Shoulder dystocia |
Preterm labour |
|
Operative delivery |
|
Monilial vaginitis |
|
Pregestational |
|
Difficult sugar control |
congenital anomalies abortions |
Ketosis |
|
Hypoglycemic attacks |
|
Diabetic vasculopathy leading to IUGR |
|
Postpartum thyroiditis |
|
Neonatal
Hypoglycaemia, hyaline membrane disease, hypomagnesemia, hyperbilirubinemia, hypocalcemia, hypertrophic cardiomyopathy, hyperviscosity syndrome, transient tachypnea,birth injuries, birth asphyxia, obesity in adulthood, hype 2 diabetes.
Management
Team care is ideal for the management of diabetic women. Best results are obtained when the patients are seen in special diabetic clinics. Team consists of physician, nutritionist, neonatologist, obstetrician and midwife.
Objective
Preconceptional
To minimize early pregnancy loss and congenital malformations in infants of diabetic mother, optimal medical care and patient education is recommended before conception.
First trimester
Careful sugar monitoring is essential for management. Individualized glucose control, parental education concerning the ensuing months of pregnancy should be explained.
Second trimester
Maternal serum alpha feto protein concentration at 16 to 20weeks is used in association with targeted ultra sound at 18 to 20weeks in an attempt to detect neural tube defects and other malformations. Maternal serum alpha fetoprotein values may be lower in diabetic women and interpretation is altered. Fetal ECHO at 22 to 24 weeks to rule out cardiac anomalies.
Third trimester
Weekly visits to monitor glucose control and to evaluate for preeclampsia are recommended. Serial ultrasound at 3 to 4 weeks intervals is performed to evaluate both excessive and insufficient fetal growth as well as amniotic volume. Hospitalization for women with uncontrolled sugar levels. A program of fetal surveillance using some of the antepartum tests between 26 to 32 weeks depending on the clinical risk factors for fetal death.
Umbilical artery doppler to study the fetoplacental flow may be done in cases with diabetic vasculopathy where IUGR is likely to occur.19,20
According to ACOG Guidelines fetal monitoring when
Begin earlier if other comorbidities are present
Start >32 week- can be based on local practice
Include amniotic fluid volume assessment due to the risk of polyhydramnios.
When to deliver?
Monitoring in GDM
Blood glucose monitoring: Once a woman has a diagnosis of GDM, routine glucose monitoring should begin. Evidence is lacking regarding the optimal frequency of testing, but the general recommendation is to monitor four times a day (ACOG 2013). This would consist of daily monitoring of fasting glucose and 1 or 2 hours after each meal. Data are insufficient regarding whether 1- versus 2-hour postprandial monitoring is superior. Postprandial glucose control is associated with better overall glycemic control and may be more predictive of maternal and fetal complications. Individuals with GDM that is diet controlled can monitor less often.
Glucose goals and other monitoring values: Observational studies show that HbA1C concentrations less than 6%–6.5% are associated with the lowest rates of fetal complications, but trials have not evaluated the risk-benefit of achieving these targets. Hemoglobin A1C concentrations during normal pregnancy fall by as much as 0.5% because of increased RBC turnover (Nielsen 2004). Furthermore, because postprandial glucose is a better indicator of risk of complications, A1C is not as useful (Table 4).21
Guideline |
Fasting glucose |
1-Hr postprandial |
2-Hr postprandial |
ACOG |
95 |
140 |
120 |
ADA |
95 |
140 |
120 |
Endocrine Society |
95 |
140 |
120 |
NICE |
95 |
140 |
115 |
Table 4 Recommended Glycemic Targets for Patients with Gestational Diabetes
Nonpharmacologic management
Nutritional therapy
Medical nutrition therapy is the cornerstone of treatment for GDM (Metzger 2007). Dietary intervention, in combination with insulin therapy as needed, reduces the risk of LGA infants, fetal macrosomia, preeclampsia, and serious perinatal complications (Landon 2009; Crowther 2005). All women with GDM should receive dietary counselling at the time of diagnosis, preferably provided by a registered dietitian or nutritionist experienced in GDM management. The goals of dietary modification in GDM are to attain the desired level of glycemic control; provide adequate weight gain, which contributes to maternal and fetal well-being; and prevent the development of ketosis.
The ACOG guidelines recommend a caloric distribution of 33%–40% carbohydrates, 20% protein, and 40% fat (ACOG 2013). Strong evidence for the optimal proportion of carbohydrates in GDM is lacking, and the Endocrine Society suggests a slightly less restrictive carbohydrate intake of 35%–45% of total calories (Blumer 2013). Other sources recommend a minimum intake of 175 g of carbohydrates per day, although this is greater than the recommended daily carbohydrate consumption (130 g) for nonpregnant women (Blumer 2013; ADA 2008; IOM 2002). Regardless of the strategy used to determine initial carbohydrate intake in GDM, adjustment of carbohydrate consumption should be ongoing and based on clinical measures such as blood glucose concentrations, ketone concentrations, and weight gain (ADA
2008).21
A typical daily meal plan for women with GDM includes three small to moderate-sized meals and two to four snacks, one of which should be at bedtime to prevent the development of ketosis overnight (ADA 2008). Meal plans should consider cultural preferences as well as desired weight gain and level of physical activity (Metzger 2007). A suggested recommendation for caloric distribution across meals and snacks consists of 10% of total calories at breakfast, 30% at lunch, 30%at dinner, and 30% divided between the snacks. In general, carbohydrate intake should be distributed throughout the day to reduce postprandial hyperglycemia, and protein should be included with all meals and snacks to promote satiety. Glucose may be more difficult to control in the morning due to the dawn phenomenon. Therefore, women with GDM may require lower carbohydrate consumption to attain desired glucose concentrations after breakfast compared with other meals. Patients with GDM should be trained in carbohydrate counting, and blood glucose concentrations should be interpreted in the context of food logs that document carbohydrate intake (Metzger 2007).22 When insulin therapy is needed, consistency of carbohydrate intake with meals and snacks is an important focus (ADA 2008).
To satisfy the various needs, the following dietary principles have been suggested as suitable for someone with GDM.23-28
National Institute for Health and Clinical Excellence for pregnant women with GDM suggests that that pre-pregnancy BMI is greater than 27 kg/m2should restrict their energy intakes to 105kJ/kg per day or less and combine this with moderate exercise of at least 30 min duration per day (Table 5).
Pre-pregnancy BMI (kg/m2) |
Total weight Gain |
Rates of weight gaina 2nd and 3rd Trimisters |
Caloric requirements |
Range, Ib |
Mean (range), Ib/wk |
Range, kcal/kg/dayb |
|
Underweight (<18.5) |
28-40 |
1(1-1.3) |
Up to 40 |
Normal weight (18.5-24.9) |
25-35 |
1(0.8-1) |
30 |
Overweight (25.0-29.9) |
15-25 |
0.6(0.5-0.7) |
22-25 |
Other (≥30.0) |
11-20 |
0.5(0.4-0.6) |
12-14 |
Table 5 Recommendations for weight gain and caloric requirements during pregnancy
Exercise
Exercise decreases peripheral insulin resistance and is an appropriate adjunctive therapy to diet for the GDM patient. Patients are encouraged to participate in exercise 30 min several times weekly. In women with relative contraindications, appropriate assessment and counselling it may be possible to allow exercise in pregnancy. Pregnant women frequently require modifications of their exercise regimen because of musculoskeletal or mechanical symptoms such as pubic symphys is dysfunction or back pain. The prescription of an upper body exercise program, for example, cycle ergometry or water based exercise programs can overcome these limitations. By including specific exercise advice in the management plan for gestational diabetes, it may help validate the women’s request for the support in both the workplace and home to allow her to meet these recommendations. As gestational diabetes is a relatively common complication, it may be practical to offer supervised group exercise such as walking groups, antenatal exercise classes or aqua aerobics.
Oral hypoglycemic agents
Generally, not recommended as they cross placental barrier and cause fetal hypoglycaemia.
Metformin
Metformin is a tablet that has been used successfully for almost 40 y to treat diabetes outside of pregnancy. It is increasingly being used during pregnancy as an alternative to or in addition to insulin.23
Metformin improves insulin sensitivity and would be expected therefore to improve tolerance in pregnancy by reducing the physiological rise in insulin resistance that occurs during pregnancy. The biguanide metformin during pregnancy has mostly been studied in the first 12 w of gestation for patients with polycystic ovary syndrome (PCOS). Starting dose of 500mg nightly for 1 week increase to 500mg twice daily, check baseline creatinine. Adverse effects include abdominal pain, diarrhoea- recommend with meals. Maximum dose is 2,500 to 3000 mg/day in two or three divided doses.
Glyburide
This sulfonylurea has been identified in the past several years as an alternative to insulin therapy for the treatment of GDM. Its primary action is to enhance insulin secretion. Starting dose is 2.5 to 2mg per day in divided doses. Upto 30mg may be necessary. Glyburide does not significantly cross the placenta. Several studies have found that glyburide serves as a suitable alternative to insulin for treatment of GDM with similar perinatal outcomes. Glyburide is a category C medication in pregnancy. Hypoglycemia may occur with all sulfonylureas, and glyburide is no exception. The incidence of hypoglycemia with glyburide ranges from 1 to 5%. The most common adverse effects are gastrointestinal and (nausea, vomiting, dyspepsia) and dermatologic (pruritis, urticaria, erythema, and maculopapular eruptions). Elevations of liver function testes have been reported, but jaundice is rare. The overall incidence of adverse effects ranges from 3.2 to 4.1 %.29
Acarbose
Acarbose has not been systemically analysed in the treatment of pregnant women with diabetes. There is a report from Mexico on six women in whom glycemic regulation was achieved with acarbose, and the pregnancies were complicated by deliveries of healthy babies. The potential unfavourable (although not proven) influence of acarbose on pregnancy could be due to the increased amount of starch in the bowels of the women treated with acarbose. The bacterial breakdown of starch leads to the accumulation of butyrate, which could increase the prostaglandin E secretion, with negative consequences on pregnancy.
Insulin therapy
The case where the patients are unable to achieve glycemic control with diet and exercise, pharmacotherapy with insulin is recommended. One recommended threshold for initiation of pharmacologic therapy requires a fasting glucose>95 mg/dl in addition to postprandial levels>120 mg/dl for 2 h or>140 mg/dl for 1 hour. Insulin therapy is safe and effective and is the gold standard against which other therapies for GDM are compared. Regular and NPH insulin, and the short-acting insulin analogues lispro (humalog) and aspart (novolog) are considered safe for safe [10] Insulin therapy increases glycaemic control. Insulin treatment for women with GDM can be done on an outpatient basis. The insulin dosage is maternal weight based. One insulin regimen doses insulin at 0.7units/kg actual body weight.29 This dosage is lower than for, non–GDM diabetic patients. This more conservative therapy is intended to prevent hypoglycaemia. After the total daily dose is calculated, two-thirds of the doses is administered before breakfast (two-thirds NPH insulin and one-third regular insulin) and the remaining one–third is broken up into 2 different doses (one–half regular insulin before dinner and one-half NPH insulin at bedtime) (Figure 3) (Table 6).

Figure 3 The American diabetes association recommends insulin for pregnant women with dm and for women with dm considering pregnancy.
Insulin type |
Onset |
Peak(hr) |
Duration(hr) |
Short acting |
|
|
|
Lispro |
<15min |
0.5 to 1.5 |
3-4 |
Aspart |
<15min |
0.5 to 1.5 |
3-4 |
Regular |
30- 60min |
2-3 hrs |
4-6 |
Long acting |
|
|
|
Isophane insulin suspension |
1-3hr |
7-May |
13-18 |
Insulin zinc suspension |
|
|
|
Extended insulin zinc suspension |
1-3hr |
8-Apr |
13-20 |
Insulin glargine |
2-4hr |
14-Aug |
18-30 |
|
1-4hr |
Minimal peak activity |
24 |
Table 6 Action Profiles of Various Insulin’s which are commonly used
Monitoring glycemic control
The success of the treatment during GDM depends on the glycemic control maintained with a meal plan or pharmacological intervention. To know the effectiveness of treatment, monitoring of glycemic control is essential:30
Glycemic index
Glycemic index is a number associated with the carbohydrate in a particular type of food that indicates the effect of this carbohydrate on the person’s blood glucose (blood sugar level) a value of 100 represents the standard, an equivalent amount of pure glucose. The GI represents the rise in a person’s blood sugar level 2 hrs after the consumption of the food. The glycaemic effects of the foods depend on a number of factors, such as the type of carbohydrate, physical entrapment of the carbohydrate molecules within the food, fat and protein content of the food and organic acids in their salts in the meal. The GI is useful for understanding how the body breakdown carbohydrates and takes into account only the available carbohydrate (total carbohydrate-fibre). It does not predict an individual’s glycemic response to a food, but can be used as tool for insulin response burden of a food, averaged across a studied population.
Low Glycemic Index: -55 Or Less- fructose, beans, small seeds, walnuts, cashews, most whole intact grains, vegetables, most sweet fruits, mushroom, tagatose.
Medium Glycemic Index: 56 TO 69- white sugar or sucrose, not intact whole wheat or enriched wheat, pita bread, basmati rice, unpealed boiled potato, grape juice, raisins, prunes, cranberry juice, banana.
High GI: 7O And above-glucose, high frucrose, white bread, most white rice, corn flakes, extruded breakfast creals, maltose, white potato (Table 7).
Fasting |
<95 mg/dl |
Premeal |
<100mg/dl |
1HR postprandial |
<140mg/dl |
2HR postprandial |
<120mg/dl |
Bedtime |
<120mg/dl |
2.00 TO 6.00AM |
60-90mg/dl |
Mean 100mg/dl |
|
HbA1C < 6% |
|
Table 7 Goals for Glycemic control in Pregnancy (ACOG practice bulletin 60) 31
Insulin management during labour and delivery recommended by ACOG (2005)
Postpartum care for diabetes
Postpartum management of diabetes depends on the severity and type of diabetes. For GDM, no immediate postpartum testing is required. Most gestational diabetes diagnosed in the third trimester resolves rapidly after delivery. Glucose tolerance test is strongly recommended for those patients at their 6weeks post-partum visit with a 2hr fasting GTT, because some women with GDM will recur in 30 to 50% of subsequent pregnancies. Because women with a history of GDM have a 7-fold increased risk of developing type 2 DM.31-34 50% of women with gestational diabetes develop overt diabetes within 20years of diagnosis of gestational diabetes. They should be advised with post-partum weight control, healthy diet, exercise, and yearly evaluation for diabetes (Table 8) and (Table 9).
|
No DM |
Impaired glucose tolerance |
Overt DM |
8HR Fasting |
<110 |
110-125 |
≥126 |
2hr after 75G Glucose load |
<140 |
140-199 |
≥200 |
Table 8 Post Partum Glucose Tolerence TEST (American Diabetes Association)
Time |
Test |
Purpose |
Post delivery(1 to 3 days) |
Fasting or random plasma glucose |
Detect persistent, overt diabetes |
Early postpartum(6 to 12weeks) |
75g 2hr GTT |
Postpartum classification of glucose metabolism |
1 yr postpartum |
75g 2hr GTT |
Assess glucose metabolism |
Annually |
Fasting plasma glucose |
Assess glucose metabolism |
Triannually |
75g 2hr GTT |
Assess glucose metabolism |
Prepregnancy |
75g 2hr GTT |
Classify glucose metabolism |
Table 9 Fifth International Workshop Conference-Recommended Assesments after Pregnancy with Gestational Diabetes
Neonatal care
The baby requires neonatal intensive care or special care as the blood glucose should be monitored for 48hours.early breast feeding is advocated top prevent hypoglycaemia. Haematocrit should be checked at 1 and 24hour. Early cord clamping during delivery prevents neonatal polycythaemia.
Contraception
No single contraception is appropriate for all women with diabetes. Barrier contraception or low dose pills can be safely used by women with recent gestational diabetes. As the oestrogen content of these pills are low, glucose control is not a problem with low dose triphasic pills. IUCD is not favourable in pregestational diabetes. Pregestational diabetic women should have their children in early reproductive years as vascular complications are likely to increase with age.
Prepregnancy counseling
This plays an important role for pregestational diabetes. To prevent early pregnancy loss and congenital anomalies, medical care should begin before pregnancy. A complete assessment of diabetic status and associated complications should be done to find out if she is fit to go through pregnancy; HbA1c should be evaluated to indicate a good glycaemic control before conception. Folate supplementation to be done 3 months before conception to reduce the risk of incidence of neural tube defects. Fundoscopy and renal function test to be done to rule out diabetic vasculopathy. Evaluation of thyroid function test should be done in type 1 diabetes as hypothyroidism is frequently enquired in these women. Those on oral hypoglycaemic agents should be switched over to insulin before conception
Steven Gabbe proposed the rule of 15 for GDM
Prevention
(Figure 4)
None.
The author declares there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.