eISSN: 2473-0815


Research Article Volume 6 Issue 1
1ULB Translational Medicine Laboratory, Belgium 2CHU Brugmann, departments of Biomedical Equipment, a, Digestive Surgery, b, Intensive Care Unit, c, Belgium
2CHU Brugmann, departments of Biomedical Equipment, a, Digestive Surgery, b, Intensive Care Unit, c, Belgium
3VN Sklifosovski Scientific Research Emergency Institute, Russia
Correspondence: Coulic Very, ULB Translational Medicine Laboratory, Avenue JJ Crocq 2, 1020 Brussels, Belgium, Tel 32-477-25-74
Received: December 12, 2017 | Published: February 1, 2018
Citation: Very C, Martin M, Dobos SA, et al. Anaesthesia influence on glycaemia and body temperatures (first results). Endocrinol Metab Int J. 2018;6(1):50-59. DOI: 10.15406/emij.2018.06.00154
Background: The negative effect of the patient’s temperature decrease is well known among surgeons and anaesthesiologists. The influence of critical situations including operation stress on glycaemia levels and evolution was also reported. The link between the two factors has remained rather unclear. Disposing a device/ADD/ allowing the continuous measure of core/deep and superficial body temperatures and their difference (dT) evolution we have tried to differentiate the role of anaesthesia and surgery in glycaemia and temperature evolution in rats and humans.
Method: 40rats were used and investigated during and immediately after anaesthesia alone and after thoracic surgery under the same anaesthesia. A group of 5 healthy not anesthetized volunteers and 5 informed consenting patients undergoing digestive surgery was also investigated. ADD was used for temperature measures, pH meter or strips - for glycaemia measure.
Results: Use of ADD device composed of 2 digital temperature captors, an analysing processor and registrar has allowed a continuous recording of the temperature regimen of the body and has shown that anaesthesia by itself can lead to significant hypothermia and to death if correction is not provided. Glycaemia levels varied in different ways: without correction they could dangerously either increase or decrease. Their decrease under normal level was a bad prognostic sign. Correlation between glycaemia and dT was not evident. Surgery has only deepened the effect of anaesthesia. In clinics the described effects were less expressed because of permanent empiric correction.
Conclusion: Anaesthesia and surgical intervention were confirmed to influence significantly glucose metabolism and energetics of the body. ADD device can be used as a testimony of temperature production and release that is of energetic balance of the organism. The dT continuous registration, besides glycaemia levels recording may be the basis for an adequate and quick monitoring of the patient’s condition.
Keywords: anaesthesia, glucose metabolism, body temperatures, energetic balance, digestive surgery
ADD: Apparatus For Diabetes Diagnosis Or Apparatus For Temperature Difference Diagnosis; BW: Body Weight; °C: Centigrade Degree; dT: Difference between Core and Superficial Temperatures; mg/dl: Milligram/Decilitre; mM/l: Milli Moles Per Litre; M±SD: Mean Value ± Standard Deviation; N: Number of Observations; Tc: Core Temperature; Ts: Superficial Temperature
The problem of temperature variations during and immediately after surgery is not new,1‒4 but up to now the per-operation registration was not easy and not recorded. Nevertheless it presents a sure interest for the patient’s condition monitoring and healing perspectives. It is also well known that acute situations in critically ill or post-operation patients have a significant influence on their glycaemia levels.5‒12 However the link between these two factors does not seem to have been established though the part of glucose utilization in the heath production of the organism is known to be about 70%.13 Recently new possibilities of approaching the investigation of the heath loss during surgery has appeared thanks to a new device called ADD (apparatus for diabetes diagnosis) which continuously registers core/deep (Tc) and superficial (Ts) temperatures of the body and their difference (Tc-Ts=dT).14,15 This device has proved to be useful in the diabetes treatment when coupled with an insulin pump, programmed by the results of dT evolution.16‒20
So it could be interesting to use this ADD for clearing the respective role of anaesthesia and surgery in temperature per operative disorders and establish a parallel with the results of glycaemia investigation. In this work we try to consider apart and differentiate the influence of anaesthesia (which can decrease the basal metabolism) from the influence of the surgical act itself, hoping to find means of the patient’s management optimization. EMIJ-06-00154-g003A&B
The experimental part of the study was conducted on 40 white laboratory rats: males, BW 350-400g, displayed in 2 equal series: anaesthesia only and anaesthesia plus surgery (Table 1). After induction with Fluorotane® (4% during 1minute/100gBW) all rats were given intra peritoneal injection of Nembutal (pentobarbital 0.075mg/100g BW), Temgesic® (0.2ml) and subcutaneous Atropin Sulfate (1%-0.2ml). Ambient temperature was 22-24°C.
Subjects |
Series |
Number |
Observation delay |
Remarks |
Rats |
Anaesthesia only |
20 |
|
Euthanasia at days 1-7 |
|
with warming/cooling |
8 |
|
|
|
without intervention |
12 |
|
|
|
Anaesthesia + Surgery |
20 |
|
|
|
thoracic |
10 |
Up to 13months |
Euthanasia at days 7-400 |
|
abdominal |
10 |
2days |
<4-48hour survival |
Total |
|
40 |
|
|
Table 1 Experimental series
In 7 cases of the first group, after 1-2hour observation warming of the animals by a lamp (air temperature up to 30°C) was started and maintained during 1hour or more depending on the animal’s reaction i.e. till the Tc reached 38°C. In 8 ases, 10% or 5% glucose solution bolus was administrated respectively per mouth (0.2mlx2) or subcutaneously (0.4ml). In 5 cases no intervention was undertaken. In the second group table warming (39°C) was maintained during the thoracic operations.
The clinical part involved 5 informed volunteers were tested without anaesthesia and 5 consenting informed patients were investigated during severe high abdominal surgery, performed under usual classic anaesthesia using Propofol or Etomidate or Ketalar as inductors and Sufentanyl for the main analgesia. During intervention they received perfusion with Hartmann solution.
Methods of investigation
Temperatures were evaluated by usual room thermometer and body temperatures by the ADD device. In its last modification the device was composed of a double temperature digital sensor, a processor and an interface for communication with insulin and glucose pumps (Figure 1). The last were not used in the present study which was only observational. The sensors were included at a 4.5cm distance from each other (to avoid temperature influence on each other) into an isolated catheter of 4mm diameter enclosed into a thin, hermetic, waterproof and one-using polyethylene membrane. When the catheter was placed in the rectum the inner sensor being at the top of the catheter was situated at the level of the deep haemorrhoidal venous plexus in human, at the hepatic angle of the rat colon. It registered the core or deep temperature (Tc) supposed to reflect thermogenesis.

Figure 1 Schema of the used device (Apparatus for Diabetes Diagnosis and CGT Complex for Insulin and Glucose Therapy). The device was used only in observation regiment (ADD).
In humans as well as in animals, the lower sensor was placed just above the external anal sphincter and registered the superficial temperature (Ts) of the subcutaneous para-rectal fat.
Preliminary thermostats tests of each sensor, as well as their coupling, at different temperature levels, provided several times at 2month intervals have shown a resolution of 1byte (0.0625°C), a precision of 0.1°C, a good reproducibility and a maximal derive between sensors registered values of 0.19bytes, that may be considered as not significant (Figure 2).
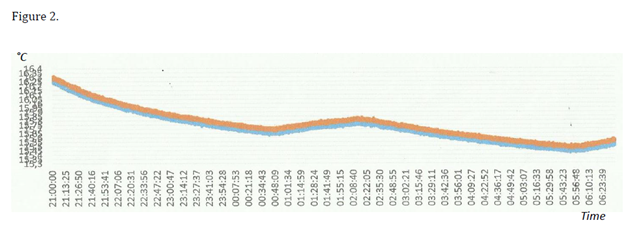
Figure 2 Example of graphic obtained when testing apparatus ADD during 9hours (from 21H till 06H of the next day). Temperatures of the two sensors have changed in the same way (error=0.19 byte, =0.003°C), so DT is stable and constant.
Blue line, deep/core temperature; Red line, superficial temperature. Ordinate–bytes (1 byte = 0.0625°C), abscissa Clock time in hours and minutes.
In all the cases (except healthy volunteers) the rectal catheter was introduced after sedation or anaesthesia. Glycaemia was measured every 30min in rats, every hour in men, either by strips (One Touch- Verio IQ, LifeScan, Switzerland) or by gasometer apparatus (ADL-90Flex) during operation in clinics. Both methods traditionally show an error of 2% of the indicated ciphers, according to the manufacturers’ documents.
For statistic evaluation Mean values ± Standard Deviation were calculated and Student criterion Td versus Tst was applied for determination of liability (p). Involvement of other not parametric statistic was considered but did not change the results. Correlation between dT and glycaemia was not systematically calculated considering the probable discordance in time of the two parameters.21,22
All the manipulations on animals were provided according to the rules of animal welfare and the local Ethics Committee (protocol N°420N). When required, euthanasia was performed by anaesthetics overdose. Clinical investigations were also allowed by the Ethic Commission of the CHU Brugmann (Brugmann University Hospital Center), Brussels.
The procedure of temperature registration was well tolerated by both humans and animals. In some rats after more than 3hours observation signs of intestinal engorgement were to be noted due to the partial obstruction by a relatively large for them catheter. However after awakening no complication was observed.
In experiments, during anaesthesia only, both temperatures rapidly decreased, Tc first, then Ts within a few minutes (Figure 3A&B). So dT remained rather stable (variation was not significant). It is to be noted that the first recorded temperatures within 10-20min after the anaesthesia initiation were already relatively low: from 33.8±0.6°C to 35.74±1.27°C in different groups considered. Sudden increase or decrease of DT could correspond respectively to awakening or to degradation of the rat condition. Decrease of Tc fewer than 32°C level because of refreshment or due to anaesthetics overdose was often irreversible (Figure 3C).
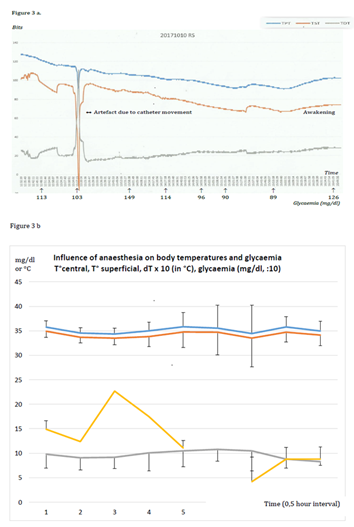
Figure 3A&B Influence of anaesthesia only on body temperatures and glycaemia in rats (examples and graphics)
Example (a) and graphic (b) of the evolution of temperatures and glycaemia during anaesthesia in rats without external intervention, issue – awakening.
Addition of a warming lamp or administration of glucose 5% or 10% subcutaneously or per os (0.2-0.4ml) had an immediate positive effect on temperatures and dT, (Figure 3D&3E).
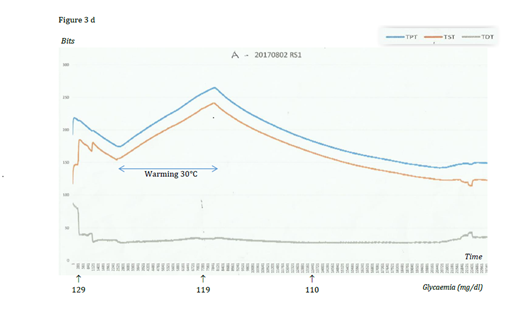

Figure 3D&E Example and graphic of the influence of warming by a lamp: quick elevation of temperatures and DT, glycaemia slightly diminishing or stable.

Figure 3F Influence of repeated glucose administrations: increase of the body temperatures, the core one first, no significant influence on glycaemia.
Core temperature – blue, superficial temperature – red, dT – green, glycaemia – orange Time on graphics in 30min intervals.
During anaesthesia only in half of the cases glycaemia increased up to pathologic levels (149-230mg/dl) and within 2-3hours decreased, often up to normal level (Figure 3B). Low glycaemia (<70mg/dl) was, as a rule, irreversible and a sign predicting a lethal issue.Warming the animal have no immediate effect on glycaemia and a glycaemia decrease was observed in some cases. It is also to be noted that initial glycaemia values in different groups did not significantly differ (N total=30, M=135.7, SD=17 mg/dl; for different groups=129.6±203; 149±18; 140±20; 125±9,44), but the error SD/M varied from 7% up to 14%.
Analogous alterations were observed after surgery* in rats, may be with a more important temperature decrease probably due to the thoracic or abdominal cavity opening, and a more significant glucose level increase (up to 350mg/l) (Figure 4 & Table 2). Interesting to note that a coming back to Tc 34°C and glycaemia 100-120mg/dl was a positive sign, even a condition, of the animal awakening and successful surgery issue (Figure 4). On the contrary, low temperatures and low glycaemia were always present before lethal issue (Table 3).
Time |
Glycaemia mg/dl |
Glycaemia ranges (mg/dl) |
Total number |
|
<100 |
>150 |
|||
Before intubation |
115.9±11.5 |
0 |
0 |
10 |
After intubation |
142±24 |
0 |
|
16 |
After operation |
143±40 |
2 |
5 |
15 |
After operation |
157±90 |
1 |
10 |
14 |
After operation |
189±138 |
1 |
9 |
13 |
After operation |
273±126 ** |
0 |
5 |
13 |
After operation |
289±86 ** |
1 |
5 |
8 |
After operation |
254±27 ** |
2 |
4 |
8 |
Table 2 Glycaemia evolution after anaesthesia, before and soon after thoracic surgery in rats (N=14)
p<0.05*; p<0.01** relatively to values obtained before intubation.
Time\Indices |
Tc (°C) |
Ts (°C) |
dT in °C |
Glycaemia (mg/dl) |
H0 († moment) |
33.11±1.12** |
31.19±1.49** |
2.08±1.09 |
90.4±33.1 |
30min before † |
33.6±0.90** |
32.2±0.83* |
1.5±0.835 |
113.4±42 |
60min before † |
34.0±0.58* |
32.8±0.93 |
2.15±1.05 |
136.7±37.5 |
90min before † |
34.57±0.60 |
32.2±0.92 |
2.21±0.48 |
157.8±32 |
120min before † |
34.88±0.28 |
32.99±0.35 |
1.99±0.30 |
121.3±13 |
Initial values |
35.6±1.25 |
34.88±2.07 |
1.932±0.953 |
134±25 |
Table 3 Decrease of glycaemia and body temperatures during the hours preceding lethal issue (N=7)
p<0.05 *; p<0.01**; relatively to initial values.
Tc, Core Temperature; Ts, Superficial Temperature; dT=Tc-Ts; †death
It was to be noted an absence of correlation between temperatures and glycaemia, except in the period of the life end, when the fall of both indices was quasi parallel (Figure 3&4).
In healthy humans glycaemia remained between 90 and 120mg/dl (SD±9 till±22); body temperatures slightly decreased but remained higher 36°C and dT also decreased but was always positive (no food supply during the 4hour session) (Figure 5A).

Figure 5A Healthy persons. DT decrease at the end of session.
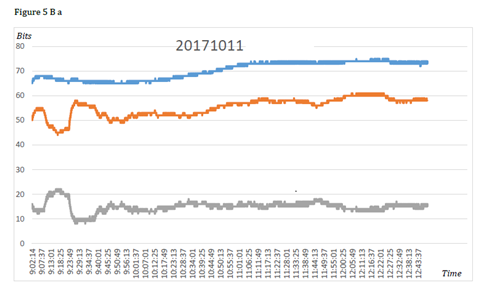
Figure 5B Examples of two different patterns (A and B) of patient’s body temperatures and dT reaction during surgical intervention.
Figure 5 Evolution of DT in humans.
In clinics, during digestive surgery, which ran without major difficulties or complications and had a favourable issue, body temperatures and dT have shown two patterns of variations. First when Tc and Ts remained at acceptable levels and dT was no less than 50bytes (0.3°C) and was always positive (2patients aged 60 and 66years). The second pattern consisted in a significant Tc decrease leading to negative dT soon after the surgery beginning and maintaining during the whole intervention (3patients aged 80-84years). For the 5patients the management conditions–air temperature, warming, adequacy of anaesthesia, and intra venous Hartmann perfusion – were analogous.
Abdominal surgery have shown the same tendencies than thoracic surgery but the survival varying between 0.5 and 1.5hour, the results have been included only partly in Table 3.
Though our data are preliminary their analysis allows some reflexions.
There is to be noted that statistic analysis of our data has shown an important variability of the investigated indices, especially glycaemia and dT, (even for control values error >10%). It cannot be explained by investigation methods weakness which marge of error was <2%, An insufficient observation number is not probable, because the total number of initial glycaemia (Gi) determinations after anaesthesia but before any other manipulation, was 30 (M±SD=137.5±17.2mg/dl) and the variations between the above mentioned global results and Gi of different observation groups were not significant (see results in experiments, §3). The conditions of observation were maximally standardized. Nevertheless the reactions of different animals began at different moments and developed with different rates, and that could be a cause of statistics difficulties, which we tried to avoid in Table 3, beginning the report from the end point. These variations could also be due to individual characteristics of the animal glucose and energetic metabolism (including glycogen reserves, functional capacities of the insulin and contra insulin system, reactivity in shock, stress or other pathologic situations), which we could not assess. The observed individual responses to the tests even performed on laboratory animal in standardized conditions deserve further study.
Body temperature measures were, at the contrary, more reliable (error <5%), nevertheless dT (Tc-Ts) variability was high (error often >10%). We know that Tc and Ts evolution was different: Ts evolution was slower than Tc one and probably depended on organ and tissue energetic needs also conditioned by the blood flow intensity (which unfortunately we could not measure). That may explain complementary dT variations of different amplitudes. Anyway, the phenomenon remains not quite clear and requires further investigation.
It is the reason why, in the analysis of our results, we have considered as well the group tendencies and the individual evaluations. We have also considered as basic control ciphers Tc >35°C, Ts >33.5°C, dT>0 and glycaemia between 90 and 140mg/dl. For bio ethics reasons all our initial ciphers were obtained after complete anaesthesia, so the temperatures were already decreased. In thoracic surgery the relatively stable rectal temperature was probably linked with constant table warming of the animals (Figure 4).
Anaesthesia is known to cause body temperature decrease.1‒4 and this was confirmed in our experiment. This effect seems to be independent on the drugs involved, which, in our work, were different in humans (mainly Sulfentanyl®) and in animals (mainly barbiturates), unless different drugs led to analogous metabolism depletion.
The anaesthesia only had also influenced the glycaemia, but the reactions were opposite: increase in the majority of the cases, decrease in some ones. This difference of reactions in animals of the same strain, sex, BW is difficult to explain except considering the preliminary endogenous glucose reserves, other parameters such as hormonal (insulin, catecholamine, glucagon) accessibility and other factors which are not easy to determine and obtain quick results. This would be confirmed by some clinical observations in ICU which have shown that the reported hyperglycaemia could correspond to different levels of blood insulin contents.12
In animals after anaesthesia and surgery, temporary hyperglycaemia was more frequent and more important, than after anaesthesia only. This was probably due to the influence of intubation and operative stress, and corresponds to literature data concerning acute critical care.5‒9
Taking into account the concomitant variations of glycaemia, Tc, Ts and dT, it was clear that when both temperatures, dT and glycaemia were falling, the vital prognosis was bad (lethal) - see Figure 3C, that confirms some literature data about negative effect of a too strong observance of glycaemic regimen.5,10,11 A marked tendency to normalization of these indices was practically a condition for anaesthesia and surgery favourable issue (Figure 3A,D,E & Figure 4A). Was it due to the end of anaesthetics effect or to adequate support of energetic balance as in Figure 3F in animal or Figure 5A. in clinics? that is still to be determined.
It was also observed that temperature reaction to condition modifications, such as warming, cooling, glucose administration, were quick (within a few minutes), whereas glycaemia changed significantly later (>Figure 3F). This confirms other literature data.19‒22 Correlation between glycaemia and temperatures was sometimes direct – at the life end, or in healthy persons, for instance. It was often inverse and not permanent (Figure 3).
The observation that glycaemia changes seemed to develop more slowly than temperature ones, supports the hypothesis that temperature measures and especially dT (Tc thermogenesis and Ts thermic “outlay”) reflect the very energetic process whereas the glycaemia, especially venous (measured in our animals) reflects the amount of remaining free, after distribution in the organs and tissues, not yet used “combustible” that is the result of the process.
From this point of view, it is possible to suggest an interpretation of some observed results. If glycaemia was high it meant that either glycogenesis was boosted – then Tc and dT must be increased, or glucose utilization is diminished and Ts decreased and dT increased. Hypoglycaemic situations might be the result either of decreased thermogenesis – then Tc was low and dT may be <0 (for instance in animals at the end of the life or in decompensated type 1 diabetic patients).23 or of increased glucose utilization by the tissues (as in hungry or tired healthy persons).16,19 and then Ts was stable or relatively high and glycaemia also kept stable. Insufficient thermogenesis might be the explanation of dT <0 in the second group of our patients. Is it possible that glucose (and insulin?) complements could correct their supposed “energetic” deficit?
As far as Tc, Ts and dT may be registered in continuum and their sensibility to internal and external condition variations seems high, the use of temperature registration during surgery (ADD device) could be useful for the early detecting the possible patient’s condition degradation and then for correction of the anaesthesia management. The start points should be maintaining Tc no less than 35-36°C and dT positive values without yo-yo phenomenon by means of automatic monitoring of glucose (and insulin?) administration as it was realized in the treatment of acute disorders of the glucose metabolism using this “energetic feedback” for monitoring insulin treatment.15‒20 The precision and resolution capacities of the ADD device having satisfied the required accuracy, thanks to digital technique and differential calculation of dT, they might warrant an adequate adaptation to individual characteristics of the patient “energetic” metabolism.
This demands confirmation in further cautious and accurate studies.
Both anaesthesia and surgical intervention were confirmed to influence significantly both glycaemia and body temperatures i.e. glucose metabolism and energetics of the organism, operative shock deepening the effects of anaesthesia.
Though the registered values of dT and glycaemia values did not correlate, at least in time, simultaneous registration of these markers during anaesthesia and surgery seems to be appropriate for the physiological change detection and understanding.
The ADD device has shown itself liable and accurate as a testimony of temperature production and release that is of the organism energetic balance.
The ADD use in per operation conditions may be useful for rapid detection of general condition degradation.
The obtained data allow to consider the possibility of using the “energetic” feedback to monitor both insulin and glucose administration for an adequate and quick modulation of the necessary correction and monitoring of the patient’s condition.
The authors express their thankfulness to Professors Ph Vanderlinden (Anaesthesiology), L Bruyninx (Digestive Surgery) for their support, Drs F Mboti (Digestive Surgery), and J Bidgoli (Anaesthesiology) for their participation to the first operations with ADD use in the CHU Brugmann. We are grateful to Ms M Leroy (IPG, Gosselies, Belgium), To Mr A Bekkouri (ULB) to Mr J-L Kempeneers for their logistic help.
The authors declare that there are no conflicts of interest.
None.

©2018 Very, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.