Advances in
eISSN: 2572-8490


Over the last decades, hydroxyapatite (HA) and its composite with biopolymer have been extensively developed and applied in biomedical application. The aim of this study is to produce a novel 3D porous HA scaffolds for bone tissue regeneration. Scaffolds with varying composition of HA, chitosan, gelatin, agarose and poly vinyl alcohol (PVA) were prepared using freeze drying method. The composite scaffold was analyzed to determine density, porosity, biodegradability, swelling kinetics, morphology and structural properties. Porosity and density of the prepared scaffolds were 85.17 to 92.21% and 0.317 to 0.495g/cm3, respectively. The swelling ability of the prepared scaffolds showed similar efficacy. FTIR analysis showed intermolecular interaction between components in the scaffold. Pore size of the developed scaffolds were measured by scanning electron microscopy and found that regardless of their composition showed adequate pore sizes ranging from 174 to 405µm. Brine shrimp lethality assay indicated that the obtained scaffolds had no cytotoxic effects, and that they had good biocompatibility. The results suggested that, these homogeneous composite scaffolds were found to be potential candidates as bone grafting materials for bone tissue engineering applications.
Keywords: hydroxyapatite, chitosan, gelatin, agarose, poly vinyl alcohol, biomedical application
Tissue engineering evolved from the field of biomaterials development and refers to combining scaffolds, cells, and biologically active molecules into functional tissues. Tissue engineering is defined as a multidisciplinary scientific branch that combines cell biology, materials science and engineering, and regenerative medicine.1 The goal of tissue engineering is to assemble such fully functional constructs that restore, maintain, or improve damaged tissue or a whole organ. This innovative technology has attracted increasing attention as an alternative strategy to treat damaged organs and tissues that cannot be self-regenerated, such as full-thickness skin burn, over critical-sized bone defects, and chronic cartilage disease. Tissue engineering aims to eliminate the disadvantages of the conventional clinical treatments associated with donor-site morbidity and scarcity in auto grafting and allografting (allografting also introduces the risk of disease and infection transmission).2 Developed as an artificial bone matrix, a tissue engineering scaffold plays an essential role in regenerating bone tissue.
The most common approach for engineering new tissues is related to cell culture and biomaterials scaffold fabrication and growth factor. The development of three-dimensional (3D) scaffold which can provide an appropriate microenvironment for tissue growth and regeneration.3,4 This development is required by tissue engineering technique. It aims at finding biodegradable and biocompatible scaffolds that can be seeded with cells for both in vitro and in vivo purposes.5 Ther fore, scaffolds must possess some special features, such as an appropriate surface chemistry, biodegradability, nontoxicity, an interconnected porous network, appropriate pore size, and shape to attain sufficient nutrient transport and cell in-growth.6 The selection of the most appropriate material to produce a scaffold is a critical step in bone tissue engineering application.7–9 Materials widely used in bone tissue engineering include agarose, PVA, chitosan, gelatin and hydroxyapatite.
Chitosan is one of the most common natural biopolymers used for bone tissue engineering. Being a linear polysaccharide, chitosan is composed of glucosamine and N-acetyl glucosamine units. It is obtained by deacetylation of Chitin. Due to several properties such as tissue compatibility, bioresorbability, antibacterial activity, and haemostatic characteristics, chitosan is a suitable material for biomedical applications.10 Moreover, the degradation products of chitosan are nontoxic, non-immunogenic and non-carcinogenic.11
Gelatin can be obtained by thermal denaturation and chemical degradation of collagen is known to benefit cell viability.12,13 In the meantime, hydroxyapatite (HA) has a chemical composition similar to human mineral tissue and can be synthesized from many natural sources with calcium-based structures, such as bovine bone, mollusk shell and coral.14,15 It is a frequent choice and possesses osteoconductive properties which demonstrated excellent cellular and tissue responses in vitro and in vivo. Together with biocompatibility, HA has chemical structures similar to bone minerals and has been used in bone graft, augmentation, and substitution.
Out of the series of natural polymers few have the unique properties required for tissue engineering, like agarose which is a polysaccharide consisting alternate repeating units of 1, 3-linked b-D-galactopyranose and 1, 4-linked 3, 6-anhydro-a-L-galactopyranose. Agarose have been used for seeding chondrocytes and when subjected to dynamic deformational loading have been demonstrated to enhance the matrix elaboration.16 The property of agarose, i.e. good mechanical strength and capacity to retain chondrocytes phenotype makes it suitable for the construction of scaffolds for tissue engineering.
On the other hand, polyvinyl alcohol, PVA, is a water soluble synthetic resin which is obtained through polymerization of vinyl acetate monomer. By hydrolysis, the acetate groups are converted in hydroxyl groups. The degree of hydrolysis in a polyvinyl alcohol reagent is controlled by this process. The polar nature of poly (vinyl alcohol) facilitates the formation of hydrogen bonds and eventual condensation with silanol groups (from developing polysilicate network) formed by hydrolysis of the silicon alkoxides.17 Moreover PVA has been proposed for controlled release systems and is employed in a variety of biomedical applications, generally being considered to be biocompatible.18,19
A polymer or combination of polymers can be fabricated into a porous scaffold through various techniques, such as sintering, salt leaching, freeze-drying, solvent casting, gas foaming, fibre meshes, phase separation, melt moulding, emulsion free drying, solution casting, etc. Sintering uses high temperatures to bond substances together as well as burn out organic material to form a porous structure.20 Salt leaching is also feasible, but is limited by the prolonged contact of particles with water and the requirement for salt removal.21 This study has chosen freeze-drying because it can form highly a porous structure and offer stability and ease of handling.22,23
This study focuses on preparing and characterizing composite scaffold synthesized from three natural-based materials-chitosan, gelatin and HA. Afterwards in combination with agarose/PVA, these three materials offer potential synergies between physical properties and bioactivity for use as bone substitutes in bone grafts, benefiting a range of surgical applications. So a well-designed three-dimensional scaffold is one of the fundamental tools to guide tissue formation in vitro and in vivo. Frontiers areas in medicine is changing rapidly from utilizing synthetic implants and tissue grafts to a tissue engineering approach that uses degradable porous material scaffolds integrated with biological cells and molecules to regenerate tissues. Therefore, the aim of the study was to formulate a suitable scaffold which can be used in in-vivo transplantation, tissue replacement, reconstruction, and regeneration.
Fabrication of scaffold
Scaffolds were fabricated according to the set of rules followed by Azami et al.24 with simple modification. Briefly, at first hydroxyapatite was weighed into a flask. Then deionized distilled water added. The mixture was stirred at room temperature for 2hours and treated by ultra-sonication until the HA powder was thoroughly dispersed in the water. Chitosan solution was prepared by mixing proper chitosan with 1% acetic acid solution. Then gelatin was added to the chitosan in 1:2ratio and the solution was agitated at 37˚C to form a chitosan gelatin solution. After stirring this mixture were contained in a 40˚C water bath. HA was added to the chitosan-gelatin and stirred for 2hours to disperse thoroughly. In another scaffold Agarose/PVA was added separately. The resultant solution was transferred to petri plates and pre-frozen at -40˚C for 24hours, followed by freeze-drying at (-50˚C~-60˚C) for 30hours to obtain porous scaffolds.
Characterization of scaffold
Porosity and density: The density and porosity of the produced 3D scaffolds were measured by liquid displacement.25 The liquid used in this study was ethanol. A sample with a known weight (W) was immersed in a graduated cylinder in a known volume of ethanol (V1) for 5min. The total volume of ethanol in the cylinder and ethanol -impregnated scaffold was V2. The ethanol impregnated scaffold was removed from the cylinder and the residual ethanol volume was recorded (V3). Each sample was measured in triplicate. The density of the porous samples (d) and the porosity of the scaffolds (Є) are expressed as follows:
Swelling ratio evaluation: Swelling ability was determined by the percentage of water absorption.26 Dry weight of the scaffold was denoted as W0. Then, porous scaffolds were immersed in PBS buffer solution with pH 7.4 at 37˚C for 24hours. Afterward, the scaffolds were taken out from PBS buffer solution and its wet weight was measured, denoted as Ww. The ratio of swelling was calculated using equation-
In-vitro biodegradability: Biodegradability of the scaffolds was characterized by in vitro study.27 The scaffolds were immersed in PBS medium containing lysozyme (10,000U/ml) at 37˚C for 1, 3 and 7days. The initial scaffold weight was denoted as W0. After calculated days, the scaffolds were washed in deionized water to remove ions adsorbed on the surface and then freeze-dried. The dry weight of the scaffold was denoted as Wt. The degradation of the scaffold was calculated using equation-
Fourier transforms infrared spectroscopy (FTIR) analysis: FTIR Spectroscopy, is an analytical technique used to identify organic, polymeric, and in some cases, inorganic materials. The FTIR analysis method uses infrared light to scan test samples and observe chemical properties. FT-IR 8400S (Shimadzu, Japan) Spectrophotometer in the range 4000-700cm-1, Resolution 4cm-1, No of scan :20 times.
Pore morphology: The pore size and surface morphology of the bio composite scaffolds were studied using scanning electron microscopy (SEM JSM-6490LV, JEOL, Tokyo, Japan). Briefly, scaffold samples were cut into small pieces and fixed on carbon tape, then dried under vacuum and platinum coated before examining under SEM.
In vitro cytotoxicity study: In vitro, cytotoxicity test of the desired sample was performed using brine shrimp lethality bioassay method as followed in Bundela et al.28 It is also a primary toxicity screening technique used as an initial screening of bioactive compounds. The brine shrimp, Artemia salina, was used as a convenient monitor for the screening. The eggs of the brine shrimp, hatched in artificial seawater (3.8% NaCl in water) and pH was adjusted at 8.5 using 0.1N NaOH under constant aeration for 48 hr to mature shrimp called nauplii. After hatching, active nauplii were collected from brighter portion of the hatching chamber and used for the assay. HA powder was dissolved in artificial seawater at 0.25, 0.50 and 0.75mg/mL concentration and was taken in Petri plates where the active nauplii were inoculated. After overnight incubation, the nauplii were counted. At the same time, 0.5mg/mL of vincristine sulfate was used to as a positive control.
General observation
In this study, three different types of scaffolds were fabricated by freeze-drying method. The choice of this fabrication technique produced scaffolds with excellent porosity that is contributed by the formation of ice crystals during freezing and its subsequent removal during lyophilization. Fabricated HA-Chitosan-Gelatin (HCG), HA-Chitosan-Gelatin-Agarose (HCGA) and HA-Chitosan-Gelatin-PVA (HCGA) scaffolds were found to be rigid structures that had a sponge-like appearance. HA particles are embedded in the complex of the Chitosan-Gelatin (CG) network matrix, and a compact block structure of the rough pore wall is formed. Even though the HA content is high, accounting for 60% of the mass of the scaffold, the HA granules still cannot be distinguished because they are ultimately covered by the organic network without any distinct interface.
FTIR analysis
FTIR data tells us more information about the interactions between HA phase and CG network films (Figure 1). The FTIR spectra of all these samples show the typical peaks of phosphate vibration at 1028-1035cm-1 , and the amide I bands at 1546, 1543cm-1 which appearance in Gel and Chi, respectively, has shifted into 1539-1544cm-1 in the HCG composites, and amino II peaks at 1408cm-1 in Chi and 1448cm-1 in Gel shift to lower wavenumber at 1400-1458cm-1, this indicated that there are a kind of chemical interactions between the calcium ions and the amino groups in CG network films or between amino groups and PO43- . The amide I (C=O bend) and amide II (C–N bend) band, respectively. Figure 1 noted that the intensity of the amide I band relatively lower than that of amide II band, which is an indication that the anionic groups (typically, C=O or carbonyl groups) of CG network film induce HA crystalline nucleation on the surface of CG network films. The amide band correspond to C=O stretch vibration revealed that C=O bond in the CG network film were weakened because of the formation of the bonding between Ca2+ ions and C=O bonds. The reflectance band at 1641cm-1 in Gel move to higher wavenumber at 1641-1651cm-1 in all HCG samples composites due to the Ca2+ ions coupled with the COO- through electrostatic interaction and the reflectance intensity increase with the enhancing of Gel content. On the other hand, peaks for CO32- vibration mode are appeared at the positions of 833-883cm-1 and 1458-1472cm-1, these observations mean that the HA crystalline were formatted on the surfaces of CG network films, but the PO43- sites of HA structure were partially substituted by CO32-groups.
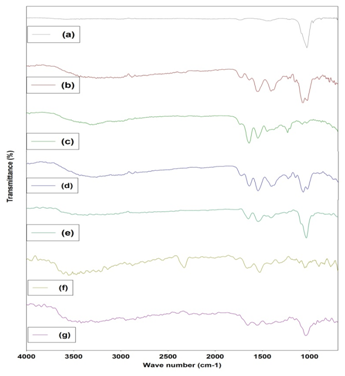
Figure 1 FTIR Spectra of HCG based scaffolds. (a) HA (b) Chitosan (c) Gelatin (d) Chi-Gel (e) HCG (f) HCGA (g) HCGP.
In HCGA scaffold, agarose was validated owing to the presence of peaks at 930cm-1 owing to 3,6-anhydrogalactose. Peak at 1077cm-1 is enhanced in the blend, which is due to the glycosidic linkages in the polymers. Few other peaks are overlapping that can be due to the similar functional groups in the polymers.
In HCGP, the broad band observed from 3100 to 3600cm-1 may be assigned to O-H stretching due the strong hydrogen bond of intra-molecular and intermolecular type.29 The presence of higher hydroxyl content in the fully hydrolyzed PVA can be seen from the broader band around 3300cm-1. The C-H alkyl stretching band17 can be observed at 2850-2950cm-1. The absorption peaks at approximately 1710-1740cm-1 and at 1090-1150cm-1 may be attributed to the stretching vibration of C=O and C-O of the remaining vinyl acetate non-hydrolyzed group of PVA polymer.17 The absorption band around 1700cm-1 arises due to the carbonyl band (C=O) of the acetate group found in partially hydrolyzed PVA polymer.
Porosity and density of the scaffolds
The porosity and density of the prepared scaffolds was measured through the liquid displacement method using ethanol. The results suggest that the porosity of the scaffolds are>85%. The porosity of the HCG, HCGA and HCGP scaffolds were measured as 92.21%±0.6%, 88.4%±0.2%, and 85.17%±0.3%, respectively (Figure 2A). Greater than 85% total porosity was observed for the polymeric scaffold, which could be an added advantage for tissue engineering purposes.30
This high degree of porosity would allow cells to migrate into and populate within the scaffold. Density of the scaffolds was measured by using liquid displacement method using ethanol. Among the samples the highest density was in HCAP sample (0.495g/cm3) and the lowest was HCG sample (0.317g/cm3) (Figure 2B). The apparent density of trabecular bone ranges from 0.14 to 1.10g/cm3. The density value increased from due to the denser packing of polymer network and as expected led to a decrease in porosity.31,32
Swelling evaluation
The ability of a scaffold to retain water is an important index to evaluate its efficacy for tissue engineering.26,33 Scaffold swelling properties have been shown to significantly influence cell behaviors such as adhesion, growth and differentiation.34,35 In this study each value of swelling test was averaged from three parallel measurements. The results of the water uptake studies are displayed in Figure 3A & 3B.

Figure 3 Swelling percentage of (a) scaffold composition on the overall water uptake at different soaking time, (b) scaffold material itself at different soaking time.
The swelling ability of scaffold was studied by immersing these scaffolds in 1×PBS solution. The results showed that there are differences in the swelling behavior among the scaffolds, where the water uptake ability of the HCG scaffold was higher when compared to other scaffold. The surface generally increases upon swelling of the scaffold, which is suitable for more cell adhesion and infiltration.
In vitro degradation studies
FTIR The aim of tissue engineering is the regeneration of tissue, with simultaneous degradation of support matrix used for the cells at a rate which can match the formation of neo-tissue. The implanted cells face the variation in the interface during the surface erosion and new surface exposure that may affect the different cellular process. Biodegradability is another important factor for biomedical scaffolds.
Degradation behaviors of porous scaffolds play an important role in the engineering process of a new tissue. In the present study, difference was observed in the biodegradation rate in HCG, HCGA and HCGP scaffolds at different time interval. However, higher degradation was observed in the HCG scaffold compared to others (Figure 4). The degradation rate of porous scaffolds affects cell vitality, cell growth, and even host response.36,37 In this study scaffolds were soaked into lysozyme containing PBS solution.
SEM analysis
The results from SEM revealed the micro architecture of the scaffolds in terms of their pore sizes, diameters, interconnectivity, and arrangements within the scaffolds. The developed scaffolds regardless of their composition showed adequate pore sizes ranging from 174 to 405µm (Figure 5).
This ideal pore range occurred predominantly in scaffolds HCG and HCGA, and as compared to HCGP that showed visible differences in pore morphology. HCG scaffolds showed well defined and interconnected pore structure, whereas the addition of agarose and PVA to the composition of HCG composite resulted in reduced pore structure. The optimum pore size for bone tissue engineering remains unclear; however, investigations that sought to identify the optimum pore size for bone tissue engineering found pore sizes ranging from 80 to 500µm to be viable.38,39 The depicted pore size enables the scaffolds to allow for cell adhesion, proliferation and also nutrient supply, which will enable proper bone tissue growth. The optical microscopic images inferred that the dispersion of the components is uniform within the scaffolding network for the fabricated scaffolds.
In vitro cytotoxicity test
The Brine Shrimp Lethality Bioassay method was used for cytotoxic effect of the composite scaffold. Scaffolds were dissolved in artificial sea water in which nauplii were inoculated. The number of death of nauplii was found to increase at higher concentration of the composite scaffold (Table 1). It may be happened owing to three reasons: cytotoxic effect of the composite scaffold, decrease of dissolved oxygen concentration of the saline water, and formation of the viscous layer on the gills of nauplii.
Sample No. |
Sample Name |
Dose |
No. of Nauplii Present |
Mortality (%) |
1 |
Positive control |
0.5 |
0 |
100 |
2 |
Negative control |
- |
30 |
0 |
3 |
HCG Scaffold |
0.25 |
30 |
0 |
4 |
0.5 |
30 |
0 |
|
5 |
0.75 |
28 |
7 |
|
6 |
HCGA Scaffold |
0.25 |
30 |
0 |
7 |
0.5 |
29 |
3 |
|
8 |
0.75 |
26 |
13 |
|
9 |
HCGP Scaffold |
0.25 |
30 |
0 |
10 |
0.5 |
28 |
7 |
|
11 |
0.75 |
25 |
17 |
Table 1 Number and percentage of mortality of nauplii after the cytotoxicity test.
In the present study, the possibility of death of nauplii owing to toxicity is very low as the number of death was nil for lower concentrations, suggesting no cytotoxic effect. Moreover, HA, gelatin and chitosan were used as parent materials of the scaffold which were both biocompatible. Hence, the most possible reason for the death of nauplii is the formation of the viscous layer on the gills of the nauplii as the highly concentrated solution of the gelatin-based scaffold led to high viscosity and thus tends to the formation of gel like structure on the gills which eventually inhibit oxygen permeability of the gills and caused death.
This study sought to identify a proper mixing ratio between HA, chitosan and gelatin for developing porous bone scaffold that could yield appropriate biodegradability, porosity, morphology and swelling properties for bone grafting. Density was increased with the addition of agarose and poly vinyl alcohol in HCG. SEM analysis showed an open pore structure of scaffolds appropriate for blood supply and cell attachment. FTIR result showed intermolecular interaction between components in scaffold. The porous bone scaffold that was developed here could be a promising bone substitute for biomedical application in the near future.
Authors would like to thank the International Atomic Energy Agency (IAEA) for their support to carry out this work as a part of Coordinated Research Project.
Authors declare that there is no conflict of interest regarding the publication of this paper.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.