Advances in
eISSN: 2373-6402


The dynamics of Cd and Zn accumulation in leaves and fruit parts (the kernel, the shell and the hull) of almond cv. Ferragnes was studied during vegetation in environmental conditions of Ravni kotari (inland coastal Croatia). A mild decrease in the concentration of Cd was observed during vegetation in leaves and fruits (hull), while this decrease was more pronounced in the shell and the kernels. Strong positive correlation was established between Cd accumulation in the kernels and the hull (r2013=0.884; r2014 =0.967), and intermediate correlation was found between Cd accumulation in the kernels and the hull. There was virtually no correlation between Cd accumulation in various parts of the fruit and leaves, which indicates an independent manner of Cd uptake in fruits and leaves. In the phenol phase of intensive growth of almond fruit the Zn concentration was the highest in the kernels (73±1.9 mg kg–1 d. wt.), followed by a rapid decrease, while continual increase was observed in the leaves, the hull and the shell. Furthermore, low negative correlation was established between the Cd and Zn concentrations in the kernels compared to the leaf, the hull and the shell. In contrast to the kernel, there is a positive correlation between the leaves and the hull (r2013=0.736; r2014 =0.811), and between the shell and the hull. Poor negative correlations were established between Cd and Zn concentrations in the leaves, the hull and the shell pointing to an independent manner of uptake of these two elements. A significant strong correlation between Cd and Zn concentrations (r2013=0.465; r2014=0.933) was established only in the kernels, due to a probable re–translocation of Zn to other parts of the fruit, and a cessation of Cd accumulation.
Keywords: almond, cadmium, zinc, fruit, leaf
Heavy metals, such as cadmium and zinc, are ubiquitous components of the biosphere.1 While Zn is an essential nutrient for living organisms, Cd is non-essential and potentially toxic for plants, animals and humans. Zinc may become toxic just like cadmium, when present at concentration higher than those required for optimal growth.2-4 Recent studies have revealed the presence of a Cd-requiring carbonic anhydrase enzyme5 and its potential role in the growth and oxidative metabolism of plants.6 Accumulation of Zn and Cd depends on the climate conditions of different plant species because the thermal effect facilitates the process of phyto extraction.7,8 Cadmium is easily absorbed and quickly transported to different plant organs.9 Along with cadmium ions, the plants absorb CdSO4 and CdCl- to a lesser extent.10 Certain plant varieties exposed to similar external Cd concentrations differ in the uptake and/or internal Cd distribution.8,11,12 The difference in Cd distribution may be due to the varying ability of individual plants to keep the absorbed Cd in its root and/or variations in the xylem supply level or Cd re-translocation into the phloem [8,13,14].8,13,14 Similarly, Cd is slowly transported from vegetative organs (leaves) to the kernels, which are used as human food.15 Some elements, such as Zn, Mn and La, can prevent Cd uptake in certain plants,3 while the Cd uptake system may be linked to the Fe uptake system, because iron deficiency further increases cadmium uptake.16 Cohen et al.17 previously showed that iron deficiency increased the Vmax for Cd influx in non-accumulator pea kernellings. In contrast, the kinetics of Zn uptake did not significantly change due to iron deficiency. In non-accumulation plants, Clements et al.18 showed that Ca transporter pathway could be involved in the uptake of Cd, albeit with a low affinity. Furthermore, Lu et al.8 showed that Cd influx into the roots decreased in hyper accumulation and non-accumulation plants, respectively, at high concentrations of Ca.
Zinc is an essential microelement for plant growth.19,20 Contrary to some other cations (Mg2+, Cu2+ and Mn2+), zinc ion (Zn2+) is not susceptible to valency changes in a plant and cannot participate in the processes of oxide reduction. Plants absorb Zn mainly as Zn2+ ion21,22 while it is still not clear to what degree they can use ZnCl+ and Zn(OH)+ sorbed on clay particles. Zn requirements of almond cultivars are generally small and the concentration of Zn in leaves and kernels among cultivars varies only slightly,23,24 while it varies significantly between different tree nuts.25 While studying the correlation of vegetative growth and reproductive activities of almonds, many researchers have established the standards for Zn concentration levels in leaves and fruits.23,24 The soil quality parameters have an influence on the elemental uptake by the nut, but the uptake and distribution of metals in the nuts are primarily dependent on the plant’s inherent controls that ensure its physiological well-being.25 Besides the importance of Zn in metabolism, its function in reducing the toxic effect of Cd on plants should also be stressed.3 The increased amount of Zn in the soil is reflected through a decreased transport of Cd from the root to the above ground parts, which is linked to a smaller diameter of the Zn atom as compared to the Cd atom. Cadmium and zinc are mostly antagonists but some studies point that their relationship can, to a certain degree, be synergistic.10,26-28
The orchard
An almond tree orchard 10 years old, fully fertile, has been chosen for the study. The necessary agro-technical measures were implemented in accordance with orchard maintenance practices. The orchard contained several almond varieties, however, the Ferragnes variety grafted on the kernelling of the bitter almond tree was chosen for this experiment, since it has shown the best agro-biological characteristics. The orchard was planted on brown forest-washed soil deposited on soft Eocene and hard Cretaceous limestone. The climate was mild Mediterranean.
Sampling time
In order to obtain quantitative results, the day after fruit set (DAFS and time 0) was conventionally considered to be the moment when the fruits had the following dimensions: length 11 mm, width 9mm, diameter 8mm.29,30
Sampling manner
Five samples were taken for each time period, and five fruit trees were selected for each sample, giving a total of 25 trees sampled at each sampling period. The fruit trees have been chosen at random and five trees were grouped for each sample of leaves and fruits. Following, the kernels, the shells and the hull were separated. In the last two procedures, due to the pre-harvest hull split, the leaf samples weren’t recorded because the leaves no longer affected the metabolism of the seed. In order to determine Cd and Zn concentrations in the leaves, kernels, the shell and the hull samples were taken beginning in May until harvest time. The samples were naturally dried and kept at a temperature of -20° C until the analysis.29,30
Sample analysis
Before the analysis, plant samples were dried to a constant weight at 105 °C and ground into fine particles of micron size. The ground plant material (1g) was burned by the wet technique, in the mixture of HNO3/HF/HC1O4. Concentrations of Cd and Zn were determined by Atomic Absorption Spectrophotometry (PU9100 X AAS).
Statistical analysis
All data were statistically analyzed using the SPSS package (Version 11.5), Analysis of variance (ANOVA) was performed on the data sets and the mean and SE were calculated for the corresponding data.
Results
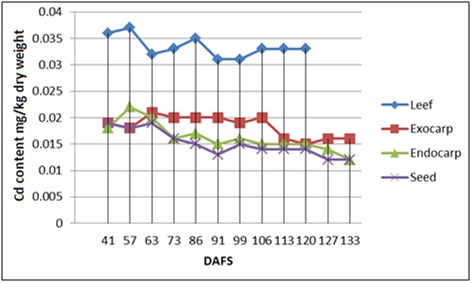
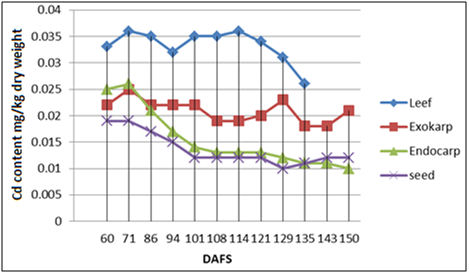
The dynamics of Cd accumulation in almond leaves and fruits: It is known that the Cd concentration in annual plant varieties is much higher than in fruit trees, proof of which is the almond tree. Generally speaking, during growth and development, the Cd concentration in leaves and hull decreased, while this decrease was more pronounced in the shell (from 0.025 ± 0.0015 to 0.011 ± 0.0006 mg kg-1d. wt.) and the kernels (from 0.18 ± 0.0014 to 0.010 ± 0.0004 mg kg-1d. wt.). It can also be seen in Figures 1 & 2 that the Cd concentration in leaves is twice as high compared to various fruit parts, specially the kernels (55-60%) close to the end of vegetation, pointing to the conclusion that Cd transport from leaves and/or xylem to the kernels is very slow. Average Cd concentrations in the studied period ranged in the leaves from 0.0333 - 0.0334 mg kg-1d. wt., in the hull from 0.0183- 0.0209 mg kg-1d. wt., in the shell from 0.0155-0.0163 mg kg-1d. wt. and in the kernels from 0.0136-0.0151 mg kg-1d. wt. A higher initial Cd concentration may be linked with a more pronounced trophic activity of the almond trees at the beginning of vegetation and/or with the levels of certain elements ( Iron for ex.) in that particular phenol phase.
Furthermore, in comparing Figures 1 & 2 it can be noticed that the Cd levels in the leaves, the hull and the shell were higher during the second year of study (2014), which can be explained by favorable climatic conditions (favorable warm temperatures during vegetation), which did not influence Cd concentration in the kernels.
The dynamics of Zn accumulation in almond leaves and fruit: The Zn concentration in almond leaves during both studied years was slightly below the optimum. During growth and development, Zn levels increased in the leaves, the hull and the shell during vegetation, while they decreased in the kernels (Figures 3 & 4). During the phenol phase of initial intensive growth of almond fruit, the Zn concentration was the highest in the kernels (73±1.9 mg kg-1d. wt.) and was followed by a rapid decrease from mid-June to the beginning of July (30± 0.4 mg kg-1d. wt.). From that point to the end of the vegetation period, Cd concentrations in the kernels continued to gradually decrease. This can partly be explained by the fact that Cd is required for auxin synthesis via the tryptophane pathway during the initial phase. A sudden decrease in Zn concentrations in the kernels, i.e. its allocation, corresponds to an increase in its concentrations in the shell, the hull-shell and the leaves where growth processes have not ended and where photosynthesis is underway. It has been established that Zn indirectly affects the intensity of photosynthesis through the ribulose-bisphosphate carboxylase. Contrary to the kernels, during vegetation Zn concentration increased in the shell, especially in 2013 (with 29 ± 0.6 mg kg-1d. wt. at the beginning up to 63± 0.9 mg kg-1d. wt. at the end) when it was inversely proportional to the beginning and end concentrations of Zn in the kernels. Smaller Zn concentration in the kernels can be reflected in poor synthesis of important compounds in the almond kernels such as sugars, proteins, amino acids, etc. In addition to its role in auxin synthesis Zn is an important activator of many enzymes and an important factor in DNA and RNA biosynthesis, protein synthesis, etc.
The observed decreasing tendency of Zn accumulation in the kernels during vegetation coincides with the increase in phosphorus accumulation, which can be the result of an antagonistic relationship between these two elements, since Phosphorus is a very important constituent of nucleic acids, enzymes, coenzymes, and phosphatides.
The correlation between Cd accumulation in the leaves, the hull, the shell and the kernels: For simpler presentation, correlations were drawn between Cd concentrations in the leaves, the hull, the shell and the kernels during the entire annual vegetation period (Tables 1 & 2). Strong positive correlation was found between the Cd concentration in the kernels and the shell for both studied years (r2013= 0884, n = 10, P < 0.01, r2014= 0.967, n = 10, P < 0.01), intermediate correlation was found between Cd concentrations in the kernels and the hull (r2013=0.513., r2014 = 0.591, n = 10, P<0.05), while poor/intermediate correlation was established between the concentrations observed in the kernels and the leaves. Intermediate positive correlation was also noticed between Cd levels in the shell and the hull (r2013=0.458; r2014=0.683, n=12, P<0.05), that between Cd levels in the leaves and the shell or the hull was poor/intermediate. It is interesting to note that positive correlation between Cd levels in the hull and the leaves was not observed, it was even negative for the year 2013. Strong positive correlations between Cd accumulation in some fruit parts and poor or negative correlations with leaf Cd levels point to an independent manner of Cd uptake in the fruit and the leaves.
Leaves |
Exocarp |
Endocarp |
Seeds |
||
Leaves |
Pearson Correlation |
1 |
-0.149 |
0.602 |
0.529 |
Sig. (2-tailed) |
- |
0.682 |
0.066 |
0.116 |
|
N |
10 |
10 |
10 |
10 |
|
Exocarp |
Pearson Correlation |
-0.149 |
1 |
0.458 |
0.513 |
Sig. (2-tailed) |
0.682 |
- |
0.135 |
0.088 |
|
N |
10 |
12 |
12 |
12 |
|
Endocarp |
Pearson Correlation |
0.602 |
0.458 |
1 |
0.884(**) |
Sig. (2-tailed) |
0.066 |
0.135 |
0 |
||
N |
10 |
12 |
12 |
12 |
|
Seeds |
Pearson Correlation |
0.529 |
0.513 |
0.884(**) |
1 |
Sig. (2-tailed) |
0.116 |
0.088 |
0 |
- |
|
N |
10 |
12 |
12 |
12 |
Table 1 Correlation between Cd accumulation in the leaves, the exocarp, the endocarp and the seeds in 2013
* Correlation is significant at the 0.05 level (2-tailed)
** Correlation is significant at the 0.01 level (2-tailed)
Leaf |
Exocarp |
Endocarp |
Seeds |
||
Leaves |
Pearson Correlation |
1 |
0.345 |
0.384 |
0.361 |
Sig. (2-tailed) |
- |
0.329 |
0.274 |
0.305 |
|
N |
10 |
10 |
10 |
10 |
|
Exocarp |
Pearson Correlation |
0.345 |
1 |
0.683(*) |
0.591(*) |
Sig. (2-tailed) |
0.329 |
- |
0.014 |
0.043 |
|
N |
10 |
12 |
12 |
12 |
|
Endocarp |
Pearson Correlation |
0.384 |
0.683(*) |
1 |
0.967(**) |
Sig. (2-tailed) |
0.274 |
0.014 |
- |
0 |
|
N |
10 |
12 |
12 |
12 |
|
Seeds |
Pearson Correlation |
0.361 |
0.591(*) |
0.967(**) |
1 |
Sig. (2-tailed) |
0.305 |
0.043 |
0 |
- |
|
N |
10 |
12 |
12 |
12 |
Table 2Correlation between Cd concentrations in the leaves, the exocarp, the endocarp and the seeds in 2014
*Correlation is significant at the 0.05 level (2-tailed)
** Correlation is significant at the 0.01 level (2-tailed)
For simpler presentation, correlations were drawn between Zn concentrations in the leaves, the hull, the shell and the kernels during the entire annual vegetation period (Tables 3 & 4). In contrast to the correlations between Cd levels in the kernels and those in the shell, the hull and the leaves, the correlations between the Zn concentrations were either poor, not found or negative for both studied years (Tables 3 & 4). This is logical since Zn concentration decreases only in the kernels during growth and development (Figures 3 & 4), and the transport of Zn is stopped due to an ongoing processes of lipid and protein synthesis for which zinc is not required but phosphorus is required (Phosphorus is an antagonist of Zinc). As a consequence, accumulation of phosphorus rapidly increases (>300%).
Leaf |
Exocarp |
Endocarp |
Seeds |
||
Leaves |
Pearson Correlation |
1 |
0.736(*) |
0.531 |
-0.101 |
Sig. (2-tailed) |
- |
0.015 |
0.114 |
0.782 |
|
N |
10 |
10 |
10 |
10 |
|
Exocarp |
Pearson Correlation |
0.736(*) |
1 |
0.533 |
-0.295 |
Sig. (2-tailed) |
0.015 |
- |
0.075 |
0.352 |
|
N |
10 |
12 |
12 |
12 |
|
Endocarp |
Pearson Correlation |
0.531 |
0.533 |
1 |
-0.363 |
Sig. (2-tailed) |
0.114 |
0.075 |
- |
0.247 |
|
N |
10 |
12 |
12 |
12 |
|
Seeds |
Pearson Correlation |
-0.101 |
-0.295 |
-0.363 |
1 |
Sig. (2-tailed) |
0.782 |
0.352 |
0.247 |
- |
|
N |
10 |
12 |
12 |
12 |
Table 3 Correlation between Zn concentrations in the leaves, the exocarp, the endocarp and the seeds in 2013
*Correlation is significant at the 0.05 level (2-tailed)
Leaf |
Exocarp |
Endocarp |
Seeds |
||
Leaves |
Pearson Correlation |
1 |
0.811(**) |
-0.395 |
-0.606 |
Sig. (2-tailed) |
- |
0.008 |
0.293 |
0.084 |
|
N |
9 |
9 |
9 |
9 |
|
Exocarp |
Pearson Correlation |
0.811(**) |
1 |
0.584(*) |
-0.539 |
Sig. (2-tailed) |
0.008 |
. |
0.046 |
0.07 |
|
N |
9 |
12 |
12 |
12 |
|
Endocarp |
Pearson Correlation |
-0.395 |
0.584(*) |
1 |
-0.163 |
Sig. (2-tailed) |
0.293 |
0.046 |
- |
0.613 |
|
N |
9 |
12 |
12 |
12 |
|
Seeds |
Pearson Correlation |
-0.606 |
-0.539 |
-0.163 |
1 |
Sig. (2-tailed) |
0.084 |
0.07 |
0.613 |
- |
|
N |
9 |
12 |
12 |
12 |
Table 4 Correlation between Zn concentrations in the leaves, the exocarp, the endocarp and the seeds in 2014
**Correlation is significant at the 0.01 level (2-tailed)
*Correlation is significant at the 0.05 level (2-tailed)
In contrast to the correlations observed for Zn levels between kernels and the other fruit parts, there is a positive correlation between Zn levels in leaves and the hull for both studied years (r2013=0.736, n=12, P < 0.05., r2014=0.811, n =12, P<0.01). This finding is plausible since these are the green parts of the tree where photosynthesis occurs and where Zn is required. Similarly as in the case of Cd, intermediate positive correlation was observed between the Zn levels in the shell and the hull (r2013 = 0.533., r2014= 0.584, n =12, P<0.05).
Table 5 reports the correlation coefficients between Cd and Zn concentrations in the leaves, the kernels, the shell and the hull. We can notice an insignificant or a low negative correlation (r2013= -0.269; r2014= -0.444) in the leaves because the increased requirements of the plant for Zn have conditioned its accumulation, and Zn, has obstructed the process of Cd uptake in the leaves to a lesser extent. Situation is similar in the case of the hull which also performs photosynthesis and where negative correlation is more pronounced (r2013= -0.451., r2014= -0.615*, P = 0.033), especially in 2014, when the Zn level during vegetation increased by about 25% and the Cd level decreased by about 10%.
Zn in the Leaves, the Exocarp, the Endocarp and the Seeds |
|||
2013 |
2014 |
||
Cadmium leaf |
Pearson Correlation |
-0.269 |
-0.444 |
Sig. (2-tailed) |
0.453 |
0.231 |
|
N |
10 |
9 |
|
Cadmium exocarp |
Pearson Correlation |
-0.451 |
-0.615* |
Sig. (2-tailed) |
0.141 |
0.033 |
|
N |
12 |
12 |
|
Cadmium endocarp |
Pearson Correlation |
-0.602* |
-0.18 |
Sig. (2-tailed) |
0.038 |
0.576 |
|
N |
12 |
12 |
|
Cadmium seeds |
Pearson Correlation |
0.465 |
0.933** |
Sig. (2-tailed) |
0.128 |
0 |
|
N |
12 |
12 |
|
Table 5 Correlation between Cd and Zn concentrations in the leaves, the seeds, the endocarps and the apocarps
**Correlation is significant at the 0.01 level (2-tailed)
* Correlation is significant at the 0.05 level (2-tailed)
The sequence of negative correlations is also present in the shell (r2013= -0.602*, P=0.038, r2014= - 0.180) especially in the year 2003 when Zn levels at the end of vegetation rose by 117% compared to those at the beginning of vegetation (29-63 mg kg-1d. wt), while Cd levels decreased by 34% (0.018 - 0.012 mg kg-1d. wt). Therefore, Zn, along with other factors, significantly affected the difficult absorption of Cd. Significant positive correlation between Cd and Zn concentrations was only present in the kernels (r2013 = 0.465., r2014= 0.933** , P = 0.000). This is especially evident in the year 2014 when Cd levels dropped by 37% (0.019 - 0.012 mg kg-1d. wt) during vegetation and Zn levels dropped by 120% (73-29 mg kg-1d. wt), due to a probable re-translocation of zinc to the other parts of the fruit and cessation of Zn accumulation.
Discussion
Root uptake of divalent cations typically exhibits two phases: apoplastic binding and symplastic uptake.3-8 During development the concentrations of Cd fell,7 chiefly in the shell and in the kernels. Consequently, in both years, the concentrations of Cd were higher in the first phases of the cycle (90-100 days after fruit germinating) and lower at the end of the cycle. The high initial Cd concentration may be linked to an intensified trophic activity of the root in the spring, as Cd enters the plant through a fraction of cellular root apoplast and gets translocated to the shoot.31 The drop in Cd concentrations in the edible part of the fruit – the kernel during vegetation, may be explained by thermal effects,7,8 intensive Fe accumulation (about 8 times more intense),23 which results in cessation of Cd absorption, an effect opposite to that of Fe deficiency (16) and/or intensified Zn and Mn absorption.23,3 Furthermore, Rauser32 concluded in his studies that Cd detoxication mechanism is connected with the synthesis of peptide and protein substances, which is in accordance with our findings.33
The Cd concentration in leaves is almost two times higher than in the kernels, the hull- hull and the shell (Figures 1 & 2), but if we examine the correlation coefficients we observe that the correlation is insignificant or non-existent, pointing to an independent manner of Cd uptake in the fruit and the leaves, that is, an insignificant and/or poor transfer of Zn from leaves to the kernels, the hull, and the shell. On occasions like these, the Cd enters the alimentary chain at lower concentrations.34 The correlations between the Cd concentrations in the kernels and the shell are positive and almost absolute (r2013= 0.884; r2014= 0.967), while those between the kernels and the hull are intermediate or significant (r2013 = 0.513; r2014 = 0.591), which points to a common manner of absorption, that is, translocation between some parts of the fruit.8 From all of the above, it appears that Cd concentration in the almond kernels in ecological conditions of Croatia is very low (0.010 to 0.019 mg kg-1d. wt). If we take into account the fact that there exists a possibility of Cd detoxication,32 due to a high protein concentration reaching 20%,33 especially the amino acid cysteine (68 mg kg-1d. wt.), then the fear from a Cd toxic effect may be completely excluded.
During vegetation, Zn concentration in almond leaves increased from 38 to 55 mg kg-1d. wt, which is below the optimum (Figures 3 & 4)19,20,23 as a consequence of edaphs and climatic conditions. In zones of temperate climate (Croatia), the physiological activity of plants and the availability of water and minerals in the soil show noticeable seasonal changes as well as changes in the climatic parameters in short time periods.7,35 Just as in the case of leaves, Zn concentration increases during vegetation in the hull (from 25-30 %), which is logical because photosynthesis also takes places in the hull. Zn is an essential and indispensable element in the synthesis of auxins, proteins via the ribulose-bisphosphate carboxylase, and has an influence on the intensity of photosynthesis. It is also an important activator of many enzymes and an important factor in the synthesis of DNA and RNA, etc.36 In the phase of intensive fruit growth (60-75 DAFS), Zn levels are the highest in the kernel, after which they rapidly drop in the kernel and proportionally increase in the shell, which points to partial re-translocation of Zn from the kernel to the shell. The decrease in Zn concentration in the kernel, also coincides with a multiple increase in Phosphorus levels (about 3.5 times),23 which may be related, since Zn and Phosphorus often act as antagonists. Furthermore, abrupt increases in Phosphorus concentration in the kernel (coupled by decreases in Phosphorus concentrations in the leaves and the shell) as well as a drop in Zn concentration take place during intensive formation and accumulation of proteins and fat, at the end of fruit growth. Zinc is no longer required in the kernel since its growth has ended, so Zn saturation and decreased Zn absorption take place (down – regulation).3 Simultaneously, Phosphorus is a necessary bio element, since we know that it is a very important constituent of phosphatides, nucleic acids, enzymes, coenzymes, phitin acid, etc.

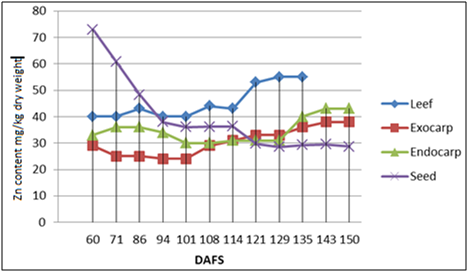
In line with the facts mentioned above (Table 5) it can be seen that there exists no correlation or only low negative correlation between the Zn levels in the kernels on one side, and the shell, hull and the leaves on the other side. Strong positive correlation exists between the shell and the hull (r2013 = 0.533, P = 0.075., r2014=0.584, P =0.046), while the correlation between the Zn levels in the leaves and the hull- hull is either high or very high (r2013=0.736, P = 0.015; r2014 = 0.811, P = 0.008), which points to a high degree of translocation in these organs.22,37
The interactions between Zn and Cd concentrations (Table 5) in the leaves, the kernels, the hull and the shell have the same tendency during both studied years. Namely, insignificant or low negative correlations observed between the uptake of the two metals in the leaves, the hull and the shell (Table 5) support the hypothesis that multiple transport systems are involved in the uptake of Cd and Zn.316,37 Negative correlation is more pronounced in the hull ( r2013= - 0.451; r2014= - 0.615*, P= 0.033) because Zn concentration increased by almost 30%, which is not surprising considering the fact the photosynthesis takes place here and that Zn is an important element for photosynthesis.23 Significant negative correlation has been observed in the shell (r2013= - 0.602*, P= 0.038), where Zn levels rose by 117%, while Cd levels dropped by 34%, pointing to an inverse proportional relationship between the uptake of these two elements.10 In the edible plant part, the kernel, there is a significant positive correlation between the levels of these two elements (r2013= 0.465; r2014 = 0.933**, P = 0.000) of both elements. An interactive relationship probably does not exist between Zn and Cd, rather, Zn gets re translocated (Figure 3 & 4) primarily into the shell (117% increase), the hull and to a lesser extent, to the leaves.23,37
The Cd levels in the kernel also decreased, which can be related to the multiple (8-fold) increase in Fe concentration 23 resulting in a cessation of Cd absorption, its translocation, and an effect opposite to that of Fe deficiency,16 and/or a strengthened Zn and Mn absorption3 and/or a Cd detoxication mechanism connected with the synthesis of peptide and protein substances,32 which matches our previous findings.33
In conclusion, the Cd concentration levels towards the end of vegetation exhibited a decreasing trend in the leaves and analyzed fruit parts, with almost a two times higher concentration in the leaves during the entire vegetative period in comparison to fruit parts. This finding points to an independent manner of Cd absorption, which may be certified by poor or no correlation between the leaves and certain parts of the fruit. Contrary to Cd concentrations, the Zn concentration levels increase towards the end of vegetation in the leaves, the hull and the shell, and decrease only in the kernels, precisely at the moment of intensive protein and lipid accumulation. In these processes, Zn does not play an important role but Phosphorus (antagonist of Zn) is required and its levels increase from then on several fold. Negative correlations between Cd and Zn in the leaves, the shell and the hull point to the obstruction of Cd uptake by Zn, because the Cd/Zn ratio is very low and favors Zn uptake and/or alternative ways of absorption. Positive correlation between Cd and Zn concentrations in the kernel may be the result of Zn re–translocation from the kernel to the shell and the hull, and a probable blockage of Cd uptake by the kernel. The answer to this question may be found in further research.
None.
The author declares no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.