Advances in
eISSN: 2378-3168


Obesity is a worldwide critical health issue that is increasing at an alarming rate reaching pandemic proportions. Unfortunately, obesity is highly related to life-threatening health conditions including cancer. Obesity serves as jet fuel accelerating the cancer hallmarks, but also important, it increases the economic burden of cancer. Although there is substantial progress in understanding the mechanisms involved in the obesity-accelerated cancer, there is still an urge to identify new targets for the development of more efficient therapeutic strategies in obese patients.
Keywords: obesity, cancer, biomarkers, adipokines, chemoresistance, caloric restriction
AKT, protein kinase b; AMP, adenosine monophosphate; AMPK, 5’-amp-activated protein kinase; CRP, c-reactive protein; mTOR, mechanistic target of rapamycin (serine/threonine kinase); IGF-1, insulin growth factor 1; IL-6, interleukin 6; JAK-STAT, janus kinase-signal transducer and activator of transcription; LKB1, liver kinase b1 or serine/threonine kinase 11; MCP-1, macrophage chemotactic factor 1; PI3K, phosphatidylinositol 3-kinase; RAGE, advanced glycosylation end-product receptor; TNF-Alpha, tumor necrosis factor alpha; TIMP1, tissue inhibitor of metaloprotease 1; WNT, wingless-type mmtv integration site family
Obesity is a critical health issue that is affecting the population worldwide.1,2 It is vital to recognize that obesity is highly related to life-threatening health conditions (e.g., cardiovascular diseases, cerebrovascular diseases, diabetes). The prevalence of obesity is increasing at alarming rate and has reached pandemic proportions. It has been proved to be among the top ten most important risk factors for all illness and causes of death in the World.3 Unfortunately, substantial epidemiological evidence suggests that obesity is a well-known independent risk factor for many cancer types (e.g., endometrial cancer, esophageal cancer, pancreatic cancer, kidney cancer, gallbladder cancer, breast cancer, colorectal cancer) across population worldwide.4–7 The American Institute for Cancer Research estimated about 25% of total cancer cases are attributed to obesity in 2014. However, the underlying biological mechanisms by which obesity accelerates cancer and promotes cancer aggressiveness are poorly understood.
The current consensus of the mechanisms involved in the influence of obesity in cancer is that they likely involve a combination of several factors including signaling pathways (e.g., PI3K/AKT/mTOR, JAK-STAT,WNT), sex hormones (e.g., estrogen), adipocytes-secreted adipokines (e.g., adiponectin, leptin), insulin, IGF-1 and inflammation (e.g., CRP, IL-6, TNF-alpha).8,9 Obesity is associated with increased circulating leptin and decreased circulating adiponectin, phenotype that is correlated in cancer risk (Figure 1).10,11 Recent studies showed that there are other obesity-induced adipokines that can be used as prognostic biomarkers and collectively can be playing an important role in the obesity-accelerated cancer aggressiveness. MCP-1, RAGE and resist in also known as adipose tissue-specific secretory factor (ADSF) or C/EBP-epsilon-regulated myeloid-specific secreted cysteine-rich protein (XCP1) is a cysteine-rich adipose-derived peptide hormone that in humans is encoded by the RETN gene have been related to metastasis,12–15 while TIMP1 has been related to chemotherapy resistance, cancer cell proliferation and cancer aggressiveness.16–18 There is no evidence showing that obesity is involved in the carcinogenesis process inducing the transformation of normal cells into malignant cancer cells. In contrast, it is clear that obesity is accelerating carcinogenesis and tumor growth enhancing cancer hallmarks.16 In other words, obesity is more like jet fuel in predisposed cancer patients.
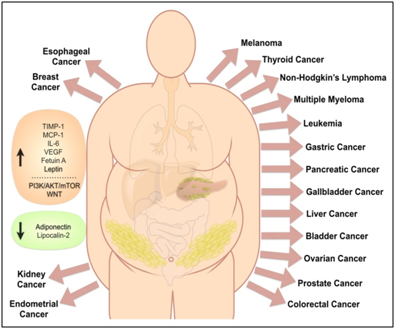
Figure 1 Obesity has been associated with increased risk of different cancer types.
Obesity is known to increase (orange box) adipokines (e.g., Fetuin A, IL-6, Leptin, MCP-1, TIMP-1, VEGF) and signaling pathways (e.g., PI3K/AKT/mTOR, WNT) that can promote cancer, and to decrease (green box) other adipokines (e.g., Adiponectin, Lipocalin-2) that are negatively correlated with cancer.
Currently, the field of cancer research has emphasized on the study of cancer metabolism to understand how the energy balance and tumor metabolism can affect tumor progression and cancer aggressiveness at the molecular level. Obesity itself is associated with systemic metabolic changes (e.g., altered lipid metabolism, glucose intolerance, insulin resistance/hyperinsulinemia related or not related to diabetes type 2) and chronic inflammation.10 Recent studies have shown that obesity can promote energy metabolism in favor of cancer cell proliferation, tumor growth and the increase of cancer aggressiveness. In contrast, disruptions of the obesity-induced energy balance via exercise, caloric restriction,19–21 or pharmacologically (e.g., metformin) can reduce tumor cell proliferation and growth.16,22,23 Indeed, obesity is associated with mild or severe (as part of diabetes type 2 comorbidity) systemic hyperglycemia and insulin resistance with hyperinsulinemia. However, studies with adipocytes co-culture model showed that mature adipocytes humorally accelerate cancer cell proliferation.16 Metformin is a biguanide, well established and effective anti-insulin resistance agent that can lowers systemic circulating glucose levels and increase insulin sensitivity. At the molecular level, metformin inhibits the PI3K/AKT/mTOR pathway, fatty acid biosynthesis and chronic inflammation via the inhibition of mitochondrial complex I and the LKB1/AMPK signaling pathway.24,25 More recently, we showed that metformin treatment disrupted lipid accumulation and adipocyte maturation during adipocytes differentiation, while metformin treatment can decrease adipokine secretion in vitro and in vivo.16 Thus, we can speculate that the obesity microenvironment is providing the essential energy building blocks (e.g., glucose, amino acids, fatty acids) for DNA synthesis and cell membrane expansion must needed for cell proliferation. However, there are many aspects of obesity-promoted cancer metabolisms that are still not well understood in order to develop more efficient targeted-therapy strategies.
In US, more than two-third of the adult population are obese (more than one-third) or overweight,26 and the estimated annual medical cost attributed to obesity has been higher than those for normal-weight people.27 However, most of previous cost-of-illness studies did not include the economic burden of obesity to cancer, thus underestimating the real financial cost of obesity to society. Adding to the economic burden of cancer, obesity has negative influences of clinical importance decreasing the efficacy of therapeutic strategies in obese cancer patient.28 These obesity-induced chemoresistance effects are more likely to be multifactorial instead of a single-driver factor. Therapeutic strategies for cancer treatment need to have in consideration the clinical relevance of obesity and implement alternative adjuvant treatments or lifestyle interventions (e.g., exercise, caloric restriction, energy metabolism disruptor such as metformin) to improve the outcome and ultimately the survival of cancer patients with obesity. There is still an urge to identify new targets for the development of more efficient therapeutic strategies to improve the outcome of obese cancer patients.
This work was supported in part by the Nathan W. Lassiter Distinguished Chair in Urology endowment funds and the Nathan W. Lassiter Distinguished Chair in Urology Dr. Bogdan A Czerniak.
The author declares no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.