Advances in
eISSN: 2377-4290


Introduction: We correlate visual acuity and microstructural changes after intravitreal bevacizumab injection in eyes with naive idiopathic choroidal neovascularization.
Methods: In this chart review study, we have 40 symptomatic eyes received an intravitreal injection of bevacizumab (1.25mg/0.05 mL) followed by additional doses based on optical coherence tomography findings, including intraretinal fluid, subretinal fluid, or pigment epithelial detachment. After their final follow up, based on visual improvement two groups were allocated, good function group: best corrected visual acuity ≥0.3 (logarithm of minimum angle of resolution) and poor function group<0.3 (logarithm of minimum angle of resolution). We analyzed best-corrected visual acuity, central retinal thickness, neovessels size (thickness and diameter) and disrupted photoreceptor layer length.
Results: Thirty one eyes (77%) showed good and nine eyes (23%) showed poor improvement in visual acuity. No significant differences at baseline were observed. Best-corrected visual acuity improved by (0.37±0.20) (logMAR) in good functional group and (0.12±0.49) (logMAR) in poor functional group and was not statistically significant (P=0.249). Change in photoreceptor disruption length was (369.09±128.4) µm in good function and (234.84±144.83) µm in poor function group and was statistically significant (P=0.038). Decrease in choroidal neovessels thickness (46.90±25.34, 32.54 ± 27.54) µm (P=0.045) and central macular thickness (105.80±22.31, 85.76±32.78) µm (P=0.043) showed significant changes at final follow up. However no significant difference in changes of choroidal neovessels diameter was observed.
Conclusion: Intravitreal bevacizumab therapy is safe and well tolerated in ICNV eyes. Restoration of photoreceptor disruption length, decrease in central retinal thickness and choroidal neovessels thickness have association with visual improvement.
Keywords: bevacizumab, idiopathic choroidal neovascularization, optical coherence tomography, photoreceptor layer, central macular thickness, visual acuity
ARMD, age related macular degeneration; CRT, central retinal thickness; D, diopters, HD-OCT, high domain optical coherence tomography; ICNV, idiopathic choroidal neovascularization; IS/OS, inner segment/outer segment; IVB, intravitreal bevacizumab; OCT, optical coherence tomography; OD, right eye; OS, left eye; POHS, presumed ocular histoplasmosis syndrome; RPE, retinal pigment epithelium; VEGF, vascular endothelial growth factor
Idiopathic choroidal neovascularization (ICNV), a unilateral ocular disease which occurs in patients younger than 50 years and diagnosed when the cause of choroidal neovascularization (CNV) is undetermined and accounts for approximately 17% of patients with CNV.1,2 Like age related macular degeneration (ARMD), compensation of CNV has been thought to be involved in the pathophysiology of ICNV but the exact mechanism is unknown.3
Optical coherence tomography (OCT) is a valuable diagnostic tool and high resolution images provide differentiation of choroidal thickness, intraretinal microstructures, including the photoreceptor inner and outer segment (IS/OS) junction and the retinal pigment epithelium. Physiologically photoreceptors play an important role in image formation and its integrity has been evaluated in various retinal diseases. It is generally believed that visible IS/OS on OCT images may be hallmark of the integrity of foveal photoreceptor layer.4
Recently several studies have clarify retinal microstructural changes, choroidal thickness and visual acuity improvement in ICNV eyes after application of anti-VEGF as treatment therapy.5 Various reports have shown that bevacizumab (Avastin, Genetech, Inc.), one of the anti-VEGF monoclonal antibodies, achieved significant visual effects in treating ICNV. These studies have mainly focus on central macular thickness, change in choroidal thickness and correlation with change in visual acuity.5,6 However no data related to IS/OS integrity and correlation with visual acuity in ICNV eyes is available to the date.
We achieved variable visual effects in our patients after intravitreal bevacizumab (IVB) therapy. We hypothesized that visual improvement might have correlation with CNV size and induced microstructural changes. We evaluate the integrity of the foveal photoreceptor layer (IS/OS layer), choroidal neovascularization size (diameter and thickness), central retinal thickness using Cirrus HD-OCT images obtained at baseline and final follow up. To our knowledge, this hypothesis has not been investigated in patients with treatment-naive ICNV according to the literature review.
This chart review study was approved by the Institutional Review Board of Xian Jiaotong Medical University. We reviewed charts of forty patients who received intravitreal bevacizumab (1.25 mg/0.05 mL Avastin, Genentech, Inc, San Francisco, CA) for idiopathic CNV between September 2013 and September 2014 and were followed up for more than 6 months in the Department of Ophthalmology. All patients were younger than 50 years and showed active CNV by ophthalmoscopy, slit-lamp biomicroscopy, fluorescein/indocyanine angiography and OCT. Active stage of ICNV was defined as leakage within the macular lesion by fluorescein/ indocyanine angiography and associated with intraretinal edema or subretinal fluid, and retinal pigment epithelial detachment based on Cirrus HD-OCT. We excluded patients with CNV because of pathological myopia (refractive error ≥-6 diopter [D] or axial length ≥ 26 mm), clinical signs of macular degeneration, angioid streaks, presumed ocular histoplasmosis syndrome (POHS), uveitis, traumatic choroidal rupture, hereditary and macular diseases and undergone previous treatment of intravitreal anti-VEGF injection, surgery, laser photocoagulation or photodynamic therapy. Best-corrected visual acuity (BCVA), slit lamp biomicroscopy and OCT were obtained at every single visit in all patients.
Both eyes pupils were dilated with tropicamide 1% and phenylephrine hydrochloride 2.5% after which transverse scan of the 6mm×6 mm area of the macular region were acquired using Cirrus HD-OCT. Images were taken by two independent trained technicians in masked fashion by (W.H. and C.N.) and measured values for each variable were averaged for statistical analyses. For the purpose of this study, subretinal fluid on OCT was defined as homogeneous hypo reflective space between neurosensory retina and RPE.7 We defined resolution of retinal fluids as absence of subretinal fluid (SRF) evaluated on OCT scans. The diameter of CNV was defined as the maximum horizontal margin that could be distinguished by OCT. Thickness of CNV was defined as the maximum CNV thickness above the retinal pigment epithelial level that could be determined by hyper-reflectivity. The integrity of the foveal photoreceptor layer was defined as the loss of the hyper reflective line and evaluated by using the photoreceptor IS/OS junction line on the HD-OCT images.8 The above defined parameters were measured manually with the virtual caliper function included in the OCT software (Figure 1). Central macular thickness was defined as mean retinal thickness of 500µm center as described in the Early Treatment Diabetic Retinopathy Study.
Statistical analyses were performed using SPSS for Windows (version 20.0, SPSS, Inc., Chicago, IL). The logarithm of minimal angle of resolution (logMAR) was calculated from decimal visual acuity for statistical analysis. Data were expressed as mean±SD. The Mann–Whitney U test was used to determine the significance of differences and dichotomous variables were analyzed by Chi square test between the two groups. Bivariate relationships were analyzed using the Pearson correlation coefficient. P values less than 0.05 were considered significant.
In this study patient age ranged from 17 to 48 years (average, 30.8 ± 8.68years) as demographics are summarized in (Table 1). At initial visit, all eyes showed presence of subretinal fluid and visual impairment associated with ICNV. After final follow up, two groups were allocated based on the improvement in visual outcome. Good function group have 31 eyes (78%), BCVA improvement ≥0.3 (logMAR) and 9 eyes (22%) BCVA improvement < 0.3 (log MAR) (Table 2).
Characteristics |
Numbers |
Mean age ± SD, years |
30.1±7.80 (range 17 to 48) |
Male: Female |
10:30 |
Eye (Right: Left) |
19:21 |
Refractive Error ± SD, D |
–2.50±2.24 (range, –4.50 to +0.30) |
Number of Injections |
2.28±1.69 (range, 1 to 4) |
Follow up Period ± SD, months |
3.60±1.20 (range, 1 to 5) |
Table 1 Demographics and clinical characteristics of patients with ICNV
SD, standard deviation; CNV, choroidal neovascularization; D, Diopters
Parameters |
Good |
Poor |
P |
Mean age |
29.85±8.92 |
30.36±6.58 |
0.839 |
Gender (male: female) |
4:17 |
6:13 |
0.815 |
OD:OS |
9:12 |
10:9 |
0.373 |
IOP, mmHg |
15.63±1.36 |
16.24±1.57 |
0.733 |
VA (logMAR) |
0.42±0.13 |
0.65±0.24 |
0.151 |
CMT, µm |
343±27.31 |
324±31.41 |
0.431 |
CNV Thickness, µm |
316±113.56 |
302±115.41 |
0.754 |
CNV Diameter, µm |
555.36±136.42 |
643.84±160.21 |
0.163 |
Disruption IS/OS Length, µm |
982.09±297.16 |
1168.85±253.92 |
0.110 |
Table 2 Comparison of baseline characteristics of patients in good visual function and poor visual function group. Data are mean±standard deviation. P relates to test of proportion from chi square test or Mann-Whitney U test as appropriate
OD, right eye; OS: left eye; IOP, intraocular pressure; VA, visual acuity; SRF, subretinal fluid; PED, pigment epithelial detachment; CNV, choroidal neo vessels; CMT, central macular thickness; IS/OS, Inner segment/outer segment
Visual acuity improved by (0.37±0.20) and (0.12±0.49) (logarithm of minimum angle of resolution) in good and poor visual function groups respectively and showed no statistical significant change (P=0.249). OCT morphological changes are summarized in (Table 3). None of the patients in our study developed systemic or ocular complications from IVB procedure (e.g., thromboembolic events endophthalmitis or retinal detachment). In present study visual acuity improvement is significantly correlated with the changes in disrupted IS/OS length, CNV thickness and central macular thickness. However no correlation was present with change in CNV diameter (Figure 2).
Parameters |
Good |
Poor |
P |
VA (logMAR) |
0.37±0.20 |
0.12±0.49 |
0.249 |
IOP, mmHg |
1.63±0.65 |
1.52±0.76 |
0.508 |
CMT, µm |
105.80±22.31 |
85.76±32.78 |
*0.053 |
CNV Thickness, µm |
46.90±25.34 |
32.54±27.54 |
*0.045 |
CNV Diameter, µm |
69.09±55.02 |
75.31±39.41 |
0.855 |
Disruption IS/OS Length, µm |
369.09±128.43 |
234.84±144.83 |
*0.038 |
Table 3 Comparison of change of variables between good and poor visual functional group
Data are mean ± standard deviation. *P value relate to linear logistic regression.
IOP, intraocular pressure; VA, visual acuity; SRF, subretinal fluid; CNV, choroidal neo vessels; CMT, central macular thickness; IS/OS, inner segment/outer segment
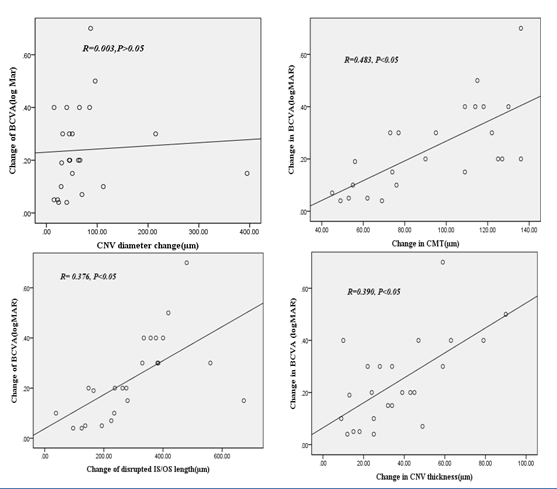
Figure 2 Correlation between visual acuity (VA) and OCT parameters. Change between the two parameters is positively correlated except choroidal neovascular diameter after application of Bevacizumab injection. (P value<0.05).
Our results are in consistence with previous studies conducted in AMD patients after application of anti-VEGF. Framme et al.9 observed CNV structural changes before and after anti-VEGF therapy in 78 eyes with neovascular AMD. They reported significant reduction in CNV thickness and showed no significant difference in CNV diameter. Similarly Byun et al.10 have treated 113 neovascular AMD eyes with intravitreal bevacizumab. They reported thicker subretinal tissues in non responders and no significant difference in CNV diameter between groups.
Previous ICNV studies have shown that decrease in CMT was related to good visual outcomes. In our study we have correlated change in CMT with best corrected visual acuity. We found that CMT decreases significantly in the good visual function group than in the poor visual group (p<0.053).11 Reported that improvement in visual acuity in eyes with ICNV was significantly correlated with decrease in CMT at first follow up after IVB.
The results of the present study showed that there was significant reduction in the status of the photoreceptor disruption length at the final follow up. Visual acuity was improved with the restoration of disrupted IS/OS line. The results of this study are consistent with those of previous studies in exudative ARMD, suggesting that the integrity of the photoreceptor layer is associated with visual outcome after photodynamic therapy or anti-VEGF therapy.7–10
After intravitreal anti-VEGF therapy, large number of patients with idiopathic CNV in this study showed good visual prognosis, which is similar to findings in previous studies.5,6 Central retinal thickness, CNV size and length of disrupted photoreceptor was reduced significantly with mean number of injections per eye was 2.28 during a mean follow-up of 3.6 months. Furthermore, all lesions were in the cicatricial stage of CNV at last follow-up, with no intraretinal or subretinal fluid by OCT imaging. No ocular or systemic adverse effects occurred after intravitreal bevacizumab therapy during the follow-up period. The treatment of idiopathic CNV is still controversial, but this study coins that intravitreal bevacizumab administration for idiopathic CNV is safe and effective.
Shortcomings of this study need to be highlighted including its retrospective nature, absence of control group and relatively small number of patients. Symptoms duration a possible prognostic factor for visual outcome was not evaluated in this study because for a few patients their information was not available. These limitations may have affected the evaluation of final BCVA and OCT parameters in this study. However, we noted a clear trend toward reduction in OCT parameters in eyes with idiopathic CNV. A clear understanding of the clinical efficacy of anti-VEGF agents will require further prospective randomized-controlled clinical trials with longer follow-up, larger patient cohorts and OCT modalities with higher resolution.
We concluded that intravitreal bevacizumab injection improve visual acuity and well tolerated in idiopathic choroidal neovascularization patients. Decrease in photoreceptors disruption length, choroidal neovessels thickness and central macular thickness have association with visual improvement.
None.
None.
The authors have no conflicts of interest to declare.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.