Advances in
eISSN: 2377-4290


Ganciclovir (GCV) is indicated for the treatment of human cytomegalo virus (HCMV) retinitis in immunocompromised patients. Sub-optimal physicochemical properties prevent GCV from reaching therapeutic concentrations in back of the eye (retina) tissue after oral and intravenous administration. Chronic high dose administration results in systemic toxicity. Local intravitreal injections suffer from poor ocular bioavailability and require repeated administration which can cause retinal detachment, retinal/vitreal hemorrhage and endophthalmitis. In the current study, we synthesized long chain acyl ester derivatives of GCV to improve lipophilicity and bioavailability. Ester conjugates (C5,C10 and C13 mono-and di-(O-acyl)) of GCV were synthesized in one step reaction following conventional esterification reaction. Purity of the novel prodrugs was determined with reversed phase high performance liquid chromatography. Conjugation of long lipid chain to GCV was confirmed with proton (1H) and carbon (13C) nuclear magnetic resonance and mass spectroscopy. Also, melting point and lipophilicity for the prodrugs and GCV were determined. MTS assay was used to assess in vitro toxicity of GCV and its long chain lipid prodrugs on human retinal pigment epithelial cell line (ARPE-19) cells. Results indicated that long chain lipid GCV prodrugs are nontoxic, safe and well-tolerated by ARPE-19 cells. These results suggest that novel long chain lipid GCV prodrugs may be further evaluated for ocular delivery and treatment of HCMV retinitis.
Keywords: characterization, cytotoxicity, 13c-nmr, ganciclovir, 1h-nmr, lipid, nmr prodrug retinal cells, synthesis
AIDS, acquired immunodeficiency syndrome; µ, Micro; mg, milligram; ppm, parts per million; EQ, equivalent; FDA, food and drug administration; GCV, ganciclovir; H, hour; HAART, highly anti-retroviral therapy; HCMV, human cytomegalo virus retinitis; IVT, intravitreal; IV, intravenous; kV, kilo volts; mins, minutes; mL, milliliter; mg mL-1, milligram per milliliter; mg kg-1, milligram per kilogram; mM, millimoles; N2, nitrogen Gas; nm, nanometers; S, seconds
Human cytomegalo virus (HCMV) retinitis is an opportunistic infection commonly affecting naturally or iatrogenically immunocompromised patients/subjects.1,2 HCMV is the leading cause of vision loss in subjects with low CD4 count (<50 cell/mm3).3,4 HCMV retinitis eventuates because of viral replication and induced inflammation in the retina. The infection initially develops in inner retinal layers and eventually spreads to other ocular tissues, such as retinal pigment epithelium leading to development of focal yellowish white granular patches, diffuse edema and retinal hemorrhage. All subjects identified with acquired immunodeficiency syndrome are recommended for HCMV diagnosis and immediate treatment initiation with anti-HCMV therapy. A significant reduction in HCMV prevalence has been noted with the advent of highly anti-retroviral therapy (HAART).5 However, there have been reports about HCMV relapse in AIDS patients during HAART therapy.6 Clinicians suggest that early diagnosis of HCMV retinitisin AIDS subjects and concurrent treatment with HAART and anti-HCMV therapy, with drugs such as ganciclovir (GCV), may reduce the incidence of vision loss.7 Similarly anti-HCMV retinitis treatment with GCV is indicated for non-AIDS/iatrogenically immunocompromised patients to prevent vision loss.8
GCV is an acyclic 2'-deoxyguanosine analogue indicated in the treatment of HCMV retinitis.9 It is the first FDA approved drug with virustatic property requiring continuous maintenance therapy to prevent HCMV relapse.10 Oral bioavailability of GCV is ~5% because of its poor absorption. Due to GCV poor bioavailability, current treatment of HCMV necessitates daily intravenous (IV) infusion of GCV.11 Chronic IV administration of GCV is associated with development of systemic toxicity, poor ocular drug permeation and emergence of viral resistance.12,13 High systemic GCV dose (5-10 mgkg-1) is associated with side effects such as neutropenia, thrombocytopenia and abnormal hepatic function. Other raised concerns include patient’s noncompliance; expensive and relative ineffectiveness. Owing to hydrophilicity of GCV, blood retinal barrier impedes deeper permeation of GCV into inner retinal tissue. Therefore, oral and IV administration of GCV cannot generate therapeutic concentrations in the back of the eye tissue (retina). There was an urgent need to avoid/minimize GCV induced systemic toxicity and improve GCV permeation. As a result intravitreal GCV inserts were developed. Local therapy mostly includes intravitreal (IVT) GCV administration (0.2–0.4 mg).14 GCV has vitreal elimination half-life of ~13 h in human15 To maintain the GCV concentration above the minimum inhibitory concentrations it necessitates frequent (2 times/week) IVT GCV administration. Chronic IVT administration is associated with vision threatening side effects such as retinal detachment, retinal/vitreal hemorrhage and endophthalmitis.16,17 Many delivery systems such as intraocular implants (biodegradable and non biodegradable), liposomes, microspheres and nanoparticles have been developed to reduce the frequency of GCV administration with varying degree of success.18−20
Strategies that improve GCV retinal permeation may significantly improve therapeutic efficacy and may also reduce the frequency of drug administration. Lipophilic acyl ester derivatization is a strategy to improve ocular bioavailability and permeation across cell membrane barriers. Earlier we reported synthesis, ocular disposition and antiviral efficacy of mono and di-acyl esters of small carbon chain conjugated GCV derivatives.21 GCV lipid prodrugs not only improved ocular bioavailability of GCV, but the ester bond hydrolysed slowly producing sustained GCV levels. Further, it was hypothesized that conjugation of long carbon chain esters may further cause slower release of GCV. Therefore, in this communication we disclose the synthesis and characterization of long chain acyl ester conjugated GCV prodrugs.
Ganciclovir (GCV) was purchased from Hubei Gedian Humanwell Pharmaceutical Co., Ltd. China. Valeric acid and 4-Dimethylaminopyridine (DMAP) were procured from Sigma Aldrich, St. Louis, MO. Dimethylformamide (DMF), N,N’-Dicyclohexylcarbodiimide (DCC) and decanoic acid were obtained from ACROS Organics, NJ. Tridecanoic acid was purchased from Aldrich Chemical Co. Inc., Milwaukee, WI.
Synthesis
Long chain lipid conjugated GCV prodrugs (C10 and C13) were synthesized following the conventional esterification reaction with catalytic amounts of coupling agents (DMAP, DCC) under inert (N2 atmosphere) and anhydrous conditions. Briefly, GCV (100mg) was dissolved in anhydrous DMF by subjecting to a steam bath followed by cooling to room temperature. Hydroxyl group of GCV was activated with catalytic amounts of DMAP (0.2 eq.). In another flask carboxyl end group of lipid (2.5 eq.) was activated with DCC (3.0 eq.) for 30mins. The activated lipid was added drop wise to GCV under inert conditions. The reactions were monitored for 48 h for the formation of prodrug with thin layer chromatography (TLC) (Scheme 1).
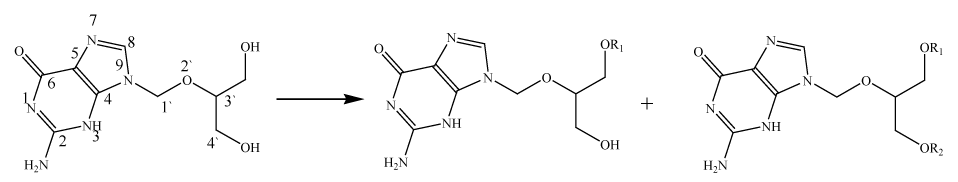
On the contrary, to conjugate C5 chain, valeric anhydride (2.5 eq.) was added to GCV. The reaction was continued for 4 h. At the end of the reaction, 2ml of water was added and solvent was evaporated overnight under high vacuum (GeneVac, UK) (Scheme 1). The crude product was subjected to flash chromatography with dichloromethane and methanol (9:1) solvent system to elute the purified products. All the lipid prodrugs were recrystallized with cold diethyl ether precipitation.
Characterization
HPLC: A high performance liquid chromatography (HPLC) system comprising Shimadzu LC 20AT pump (Shimadzu, Lenexa, KS), equipped with a fluorescence detector (Perkin Elmer, Series 200 fluorescence detector, Shelton, CT) and a reversed-phase C12 column (4μm, 250mm × 4.6mm, Synergy-max, Phenomenex, Torrance, CA) was utilized. Mobile phase comprised of 15 mm phosphate buffer (pH 2.5) and 35% acetonitrile pumped at a flow rate of 1 mlmin-1. All samples were analyzed at an excitation wavelength of 265 nm and at an emission wavelength of 380 nm.
Mass spectrometry: Mass of GCV and its lipid prodrugs (mono- and di-(O-acyl) conjugated)was determined with MDS Sciex API 3200 Triple Quadruple linear QTrap mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA)by infusing the solution with a positive ion source for detection. The compounds were dissolved in HPLC grade methanol and injected at a flow rate of 15µl min-1 into the electrospray source with a Hamilton 22 syringe pump. The mass spectra were acquired at 5 s per scan. The capillary temperature of 250°C and spray voltage of 3.5 kV were applied. Dry nitrogen was used as sheath and auxiliary gas for analysis.
Melting point and partition coefficient: Melting point for prodrugs was determined with open capillaries using melting point instrument (MEL-TEMPII laboratory devices, USA). Briefly, a small quantity of GCV or purified lipid prodrug was transferred to glass capillary tubes and was allowed to settle at the bottom of capillary tube, by tapping. The temperature range where the solid GCV or prodrug began to melt and completely turned to liquid was recorded as melting point. Octanol/water partition coefficient was determined with ACD labs software.
NMR spectroscopy: All the synthesized prodrugs spectra were recorded on Varian 400 MHz proton (1H) and carbon (13C) nuclear magnetic resonance (NMR) spectrometer (Varian, USA)in deuterated dimethyl sulfoxide (DMSO d6). Tetramethylsilane (TMS) served as internal standard and the chemical shifts are expressed in parts per million (ppm) relative to TMS.A range of 5000 – 6000 scans was accumulated for each spectrum.
Cell culture: In vitro cytotoxicity of novel lipid prodrugs was evaluated with Cell Titer 96® Aqueous Non-Radioactive cell proliferation assay kit (Promega, Madison, WI)on human retinal pigment epithelial cell line (ARPE-19 was purchased from American Type Culture Collection (Manassas, VA) at passage number 21). ARPE-19 was cultured and maintained as reported previously [22]. Briefly, ARPE-19 cells were grown in a culture medium containing DMEM supplemented with 10% (v/v) FBS (heat inactivated), 29 mM NaHCO3, 20 mM HEPES, 100 mg of penicillin and streptomycin each, and 1% nonessential amino acids at pH 7.4. Cells were grown at 37°C, in a humidified atmosphere of 5% CO2 and 90% relative humidity. The growth medium was replaced with fresh medium every other day. Cells with passage number 25 were cultured in flasks and harvested at 80-90% confluency with TrypLE™ Express (a superior replacement for trypsin) (Invitrogen, Carlsbad, CA, USA). Cells were then plated in 96-well plates at a density of 10,000cells/well and utilized for studies.
Statistical analysis: Data analysis for in vitro experiments was conducted at least in quadruplicate (n=4) and the results are expressed as average ± standard deviation (SD). Statistical comparison between the positive control and the experimental results was performed with Student’s t-test. A p-value of <0.05 was considered to be statistically significant.
Synthesis
The long carbon chain alkyl (O-acyl) mono and diesters of GCV were prepared in single step reaction following earlier described protocol from our laboratory.23 Scheme 1 shows the general method for GCV lipid prodrug synthesis. The reaction yield varied with carbon chain length. Mono (O-decyl) conjugated GCV prodrug yield was found to be higher relative to low carbon (C5) and long carbon (C13) chain (Table 1).
|
Drug/Prodrug |
R1 |
R2 |
%Yield |
Theoretical Molecular Weight |
Observed [M+1]+ or [M+Na]+ |
Melting Point (°C) |
Clog P |
% Purity |
|
Ganciclovir* |
H |
H |
- |
255.1 |
256.2 |
250-252 |
-0.03 |
99.5* |
|
GCV-mono-C5 |
n-C4H9CO |
H |
19 |
339.35 |
340.5 |
224-226 |
-0.1 |
97.5 |
|
GCV-di-C5 |
n-C4H9CO |
n-C4H9CO |
78 |
423.21 |
424.5 |
220-222 |
1.61 |
99 |
|
GCV-mono-C10 |
n-C9H19CO |
H |
27 |
409.48 |
410.5 |
216-218 |
1.98 |
98 |
|
GCV-di-C10 |
n-C9H19CO |
n-C9H19CO |
70 |
563.73 |
564.7 |
198-200 |
5.79 |
98.5 |
|
GCV-mono-C13 |
n-C12H25CO |
H |
23 |
451.56 |
452.4 |
212-214 |
3.24 |
99.5 |
|
GCV-di-C13 |
n-C12H25CO |
n-C12H25CO |
74 |
647.89 |
648.9 |
166-168 |
5.84 |
99 |
Table 1 Characterization of ganciclovir (GCV) lipid prodrugs
*Ganciclovir (GCV) purity as reported by the manufacturer (Hubei Gedian Human well Pharmaceutical Co., Ltd. China)
Valeric acid reacted vigorously with GCV resulting in a high yield of di-(O-acyl) conjugated GCV lipid prodrug. On the other hand, the reaction yield for mono-(O-acyl) valerate GCV was lower. We optimized the yield to be ~19% at 4h. On the contrary, the reactions for mono (O-acyl) decanoic (C10) and tridecanoic acid (C13) GCV conjugates were slow. The product yield was low for di-(O-acyl) ester GCV lipid prodrug (C10 and C13). In order to improve the yield for C10- and C13-di-(O-acyl) ester conjugated GCV, the reaction was reinitiated with addition of DCC and DMAP which improved the yield with time (48h). Low carbon chain acid reactions were relatively faster resulting in diester prodrug relative to long carbon chain acids. The rationale behind synthesizing these ester prodrugs is the presence of esterases in the vitreous body24 which aid in cleaving the ester bond causing slow release of GCV. The retention factor (Rf) for the novel prodrugs was determined with TLC. The Rf for C5 conjugated mono- and di-(O-acyl)-GCV prodrugs was 0.707 and 0.323, respectively. Rf for C10 conjugated mono- and di-(O-acyl)-GCV prodrugs were calculated to be 0.615 and 0.2323, respectively. Similarly, the Rf for tridecanoic acid (C13) conjugated mono- and di-(O-acyl)-GCV prodrugs was determined to be 0.5384 and 0.108, respectively. Ascending in carbon number lower the retention factor for both to mono- and di-(O-acyl) GCV derivatives. This result indicates that with increase in carbon number the elution of GCV prodrug is faster, i.e. di-(O-acyl) GCV prodrugs>mono-(O-acyl) GCV prodrug. Within the mono- and di-(O-acyl) series the trend was C13>C10>C5 indicating higher carbon number conjugated lipid prodrug eluted faster than lower carbon number GCV prodrug.
HPLC, melting point and lipophilicity
Purity of the synthesized prodrugs was determined with RP-HPLC method. The purity of eluted compounds was compared with that of the parent GCV molecule. The retention times for mono- and di-(O-acyl) GCV derivatives were different. Di-(O-acyl) derivatives generated a longer retention than mono-(O-acyl) GCV lipid prodrugs. The recrystallized lipid derivatives were of high purity and the results are presented in Table 1. The melting point of the compounds was found to be lower than GCV. The melting points for the derivatives are lower with rise in conjugated carbon number. The melting point range was quite narrow indicating the purity of the product. For the mono-(O-acyl) derivatives (C5, C10 and C13) melting point was lower than GCV. While for the di-(O-acyl) derivatives (C5, C10 and C13) the melting points were further lower than mono-(O-acyl) derivatives (C5, C10 and C13) (Table 1). These results indicate that increase in alkyl group conjugation lowered the melting points.
Partition coefficient of the prodrugs was calculated with the help of ACD labs software. Results indicate that ascending carbon chain length in the diesters long lipid conjugation improved the lipophilicity of the prodrugs. There was a large increase in calculated octanol/water partition coefficient (logP) with conjugation of tridecanoic acid to both the hydroxyl groups of GCV (Table 1). These results suggest that the conjugation of lipid chain to GCV significantly enhances lipophilicity which may aid in improving GCV bioavailability along with slow release of GCV.
Mass spectrometry
All the long chain lipid GCV prodrugs and GCV were subjected to molecular weight analysis in positive mode with mass spectroscopy. Analysis revealed GCV and all its derivatives as proton adduct [M+1]+except C13-mono-(O-acyl) derivative which was identified as a sodium adduct [M+Na]+ (Table 1). Figure 1 shows the broad mass spectra for C13-mono-(O-acyl) GCV derivative (100 ng mL-1) with a mass of 452.4 Dalton and an intensity of 7.4 X 106 cps. All theoretically calculated masses for long chain lipid derivatives were in agreement with mass spectroscopy results (Table 1), indicating that all compounds were stable.
Nuclear magnetic resonance
The proton NMR spectral data and assignments for mono- and di-(O-acyl) derivatives were calculated in comparison to the parent compound, GCV (Table 2).23Figures 2A & Figure 2B shows the proton NMR for GCV and GCV-C5 conjugated lipid prodrugs. As can be seen in the spectra for GCV prodrug (Figure 2A), we do not observe any alkyl resonance peaks downstream, i.e. between 0 ppm and 1.9 ppm. But, GCV-C5 exhibits the proton peaks corresponding to lipid chain conjugated to hydroxyl group of GCV (Figure 2B) which on integration determined the corresponded to proton number in the lipid chain. Similar proton NMR spectra were collected for other prodrugs (GCV-C10 and GCV-13). Chemical shifts in the resonance peaks for the lipid derivatives were evident relative to GCV. Conjugation of aliphatic carbon chain (C5, C10 and C13) deshielded proton NMR chemical shifts of carbon (CH2OCO) to which these groups are conjugated and the β-situated (OCH) protons. All other resonance peaks remained same to that of GCV. Spectra were similar for mono- and di-(O-acyl) derivatives of GCV but the total number of protons calculated was double in number for di-(O-acyl) GCV lipid derivatives. 13C NMR data for all prepared GCV lipid conjugates is presented in Table 2. The ester conjugated carbon (RCOO) resonance peak was evident in all the lipid prodrugs except the parent GCV molecule (Table 2). These results indicate that the long carbon chain is conjugated to hydroxyl group of GCV.
|
Carbon |
GCV (23) |
GCV-mono-C5 |
GCV-di-C5 |
GCV-mono-C10 |
GCV-di-C10 |
GCV-mono-C13 |
GCV-di-C13 |
|
2 |
153.78 |
153.78 |
153.76 |
153.78 |
153. 82 |
153.78 |
153.78 |
|
4 |
151.27 |
151.27 |
151.22 |
151.27 |
151.33 |
151.27 |
151.27 |
|
5 |
116.4 |
116.21 |
116.21 |
116.21 |
116.51 |
116.21 |
116.21 |
|
6 |
156.87 |
156.72 |
156.7 |
156.72 |
156.78 |
156.72 |
156.72 |
|
8 |
137.62 |
137.56 |
137.52 |
137.56 |
137.56 |
137.56 |
137.56 |
|
NCH2O, 1` |
71.48 |
71.21 |
71.25 |
71.21 |
71.25 |
71.21 |
71.21 |
|
CH2OH, 5` |
60.9 |
60.23 |
60.2 |
60.22 |
60.27 |
60.29 |
60.2 |
|
CH2O, 4` |
60.9 |
63.1 |
63.07 |
63.1 |
63.1 |
63.09 |
63.1 |
|
CHO, 3 |
80 |
76.79 |
76.77 |
76.79 |
76.79 |
76.7 |
76.79 |
|
RCOO |
13.27 |
13.25 |
13.85 |
13.87 |
13.99 |
13.95 |
|
|
17.64 |
17.66 |
22.01 |
22.02 |
22.09 |
22 |
||
|
34.92 |
34.93 |
24.37 |
24.29 |
24.3 |
24.32 |
||
|
35.63 |
35.64 |
28.33 |
28.39 |
28.29 |
28.41 |
||
|
28.45 |
28.47 |
28.63 |
28.72 |
||||
|
28.35 |
28.52 |
28.92 |
28.92 |
||||
|
28.64 |
28.63 |
29.04 |
29.04 |
||||
|
31.21 |
31.2 |
31.3 |
31.3 |
||||
|
33.13 |
33.13 |
33.15 |
33.15 |
||||
|
RCOO |
170.3 |
170.6 |
173 |
172.2 |
172.6 |
172.6 |
Table 2 13C NMR data for the acyl ester prodrugs of ganciclovir
Cell culture
To evaluate the cytotoxic effects of long chain lipid GCV prodrugs MTS assay were performed on ARPE-19 cells for 24 h. Percent viable cells were compared with of negative control (culture medium) (Figure 3). DMSO (10%) served as positive control and reduced the percent cell viable to 35%. ARPE-19 cell viability after exposure to our novel long chain mono- and di-(O-acyl) GCV prodrugs was comparable to that of negative control. The result indicates that exposure of prodrugs to ARPE-19 cells for prolonged period did not induce any toxicity and the compounds were well-tolerated. Results from this study clearly suggest that our novel long chain lipid prodrugs do not cause any cytotoxicity and are safe for ocular application.

In summary, we have successfully synthesized long chain lipid GCV prodrugs (mono- and di-(O-acyl)). These lipid prodrugs are efficiently eluted and separated with flash chromatography. HPLC purity tests demonstrated that compounds are of high purity. Also, the partition coefficient and lipophilicity number are improved with ascending in conjugated carbon chain number. Further, cytotoxicity analysis demonstrated the prodrugs to be safe and nontoxic to ARPE-19 cells. These prodrugs need to be further evaluated for ocular delivery and treatment of HCMV retinitis.
This study has been supported by NIH grants R01EY09171-16 and R01EY010659-14. Also, we would like to thank Dr. Nalin Chandrasoma, Deekirikewage N. (UMKC) for his help with NMR instrument.
Author declares that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.