MOJ
eISSN: 2381-182X


Research Article Volume 5 Issue 1
1Department of Food and Nutrition, Providence University, Taiwan
2School of Medical Instrument and Food Engineering, University of Shanghai for Science and Technology, China
3MediFood Research Center, Taiwan
Correspondence: Phoency FH Lai, School of Medical Instrument and Food Engineering, University of Shanghai for Science and Technology, Shanghai 200093, PR China, Tel +86-18516077898
Received: August 31, 2017 | Published: September 18, 2017
Citation: Lai PFH, Sun TC. Optimizing extraction process and characterization of antioxidant ingredients from chlorella sorokiniana. MOJ Food Process Technol. 2017;5(1):202-210. DOI: 10.15406/mojfpt.2017.05.00114
This study was to investigate optimized extraction conditions for Chlorella sorokiniana (C. sorokiniana) water extracts with antioxidant functionality and potential key compounds involved. A 2-factor, 5-level response surface methodology was employed using extraction temperature (40-100°C) and time (0.5-6h) as factors. It is indicated that, among the C. sorokiniana extracts examined, the maximal value in yield was 18.0% (w/w) on biomass basis; in 75% ethanol solubility of water extract (WS-E75S%, the potentially major antioxidant fraction), 38.0% (w/w) on extract basis; in total phenolic content (TPC), 3.17 GAEmg/g on extract basis. The highest antioxidant activities were shown by a 50% DPPH× scavenging concentration (SC50)=7.36mg/mL, 50% Fe2+ chelating concentration (CC50,)=10.4mg/mL, and reducing power increment per unit concentration=0.044mL/mg. By statistical analysis with RSREG program, the obtained polynomials for yield, WS-E75S%, SC50 and CC50 as a function of temperature and time could explain 78.9-82.2% of data variations. An optimal extraction condition was concluded at 100°C for 1h, to give high values in all yield, WS-E75S%, and antioxidant activities (i.e. low SC50 and CC50 values). In WS-E75S, the major compositions were likely nucleic acids and their analogues with ethylene structure, accompanying with detectable amounts of possibly polyunsaturated fatty acids, fatty alcohols, or phytols with acyl dienes, buta-1, 3-diene or ethylene structure. Besides these Phytochemicals, the water extract contained carbohydrates of mainly glucose and ribose (52.4 and 25.9mol%, respectively), followed by galactose and rhamnose, and two molecular fractions. Conclusively, Chlorella water extract at optimally 100 °C for 1h could yield~18% (w/w), contain WS-E75S~37% (w/w) and have statistically predicted SC50 ~3.0mg/mL and CC50 ~11mg/mL.
Keywords: chlorella sorokinian, extraction, antioxidant, DPPH scavenging, response surface design
Ara, arabinose; CC50, 50%- Fe2+ chelating concentration; CGF, chlorella growth factor; ChA, chlorogenic acid; sorokiniana, chlorella sorokiniana; DPPH, 1,1-diphenyl-2-picryl hydrazyl free radical; ECG, (-) epicatechin gallate; EDTA, (-) ethylenediamine tetraacetic acid; EGCG, (-) epigallocatechin gallate; EtOH, ethanol; F1, large molecular weight fraction; F2, small molecular weight fraction; GA, gallic acids; GAE, gallic acid equivalent; Gal, galactose; GCG, (-) gallocatechin gallate; Glc, glucose; HPACE, high performance anion exchange chromatography; HPLC-DAD, high performance liquid chromatography with a photodiarray detector; HPSEC, high performance size exchange chromatography; Man, mannose; Mw, weight-averaged molecular weight; Rha, rhamnose; Rib, ribose; SC50, 50%-scavenging concentration; TPC, total phenolic content; Vit E, a-tocopherol; WS100, water extract at 100°C for 1h; WS-E75S, 75% ethanol soluble of water extract
Microalgae Chlorella (Chlorophyceae), usually C. vulgaris, C. pyrenoidosa, and C. sorokiniana (previously named as C. pyrenoidosa), have been long consumed as nutritional supplements due to containing significant amounts of high-quality proteins with excellent amino acid profiles, polyunsaturated (W-3) fatty acids, antioxidant ingredients (e.g. b-carotene, astaxanthin, and chlorophylls), nucleic acids, vitamins, hemagglutinins, starch, and dietary fibers.1,2 Water extracts from C. pyrenoidosa in Japan are regarded as Chlorella growth factor (CGF) for human or animals.3 Oral administrations of Chlorella powder, water extracts, proteoglycans or glycoproteins isolates are reported effective for weight management, lipid metabolism control,3 increasing resistance to Listeria infection by preferentially augmenting Th1 responses and antibody levels,4-7 accelerating dioxin excretion,8 inhibiting hepatocarcinogenesis,9 and boosting immunoactivities.6,7 Accordingly, C. pyrenoidosa hot-water extracts (CPE) are patented as RespondinTM as a proprietary immunomodulator.7,10 Polysaccharides from CPE are reported to activate macrophages via Toll-like receptor 4.11 Besides, lipid extract from C. sorokiniana has been found rich in w-3 and w-6 polyunsatured fatty acids and effective to improve short-term memory in rats.12
Recently, research focuses have been on new combined processes and condition optimization for producing high value products from both Chlorella biomass and residues,1 e.g. immunomodulator glycoproteins or polysaccharides,13,14 antioxidant ingredients,15 glycosidase inhibitors [personnel communications], polyunsaturated (W-3) fatty acids,16 lutein, carotenoids, hydrogen,1 and bioethanol.17 Where Chlorella water extracts and their immuno stimulatory biopolymer isolates are drawing great attention to enhance their functionalities in anti-ageing (anti-radicals) for skin scare or boosting immunological systems for health purposes. Designing cost-effective processing conditions and looking for chemical indices for quality control are crucial for commercialization of functional resources such as Chlorella algae. Accordingly, optimizing extraction conditions for Chlorella sorokiniana extract with high antioxidant activities and potential chemical indices for quality control were investigated in this study. Antioxidant activities in scavenging abilities on DPPH × radicals and Fe2+ ions and reducing power were especially concerned. Antioxidant phytocompounds in Chlorella water extracts were identified to a great extent by high performance liquid chromatography-photo di array detector (HPLC-DAD), which were not yet discovered in literature.
Materials
Chlorella sorokiniana (previously named as C. pyrenoidosa) powder was gifted from Taiwan Chlorella Manufacturers, Ltd. (Taipei, Taiwan). It was prepared by high-pressure spray drying and possessed partly broken cell walls for facilitating extraction. Chemical standards used (e.g. chlorogenic acid (ChA), (-) epicatechin gallate (ECG), (-) epigallocatechin gallate (EGCG), (-) gallocatechin gallate (GCG), ethylenediamine tetraacetic acid (EDTA), gallic acids (GA),a-tocopherol (Vit E), and monosaccharides) were purchased from Sigma-Aldrich Co. (USA). Folin & Ciocalteu’s phenol reagent, acetonitrile, salts, acids, and ethanol were from Merck Chemical Co. (Germany) or Wako Pure Chemical Industries, Ltd. (Japan).
Extraction of Chlorella ingredients
Chlorella powder was dispersed in 500 mL distilled water (Chlorella: water: 1:50 w/w) in a 1-L screw-capped Erlemeyer flask and then put in a preheated water bath for extraction. The independent factors for extraction, i.e. temperature (T) and time (t), were set according to the 2-factor, 5-level central composite experimental design indicated in Table 1 and the extraction conditions in Table 2. The ranges for temperature and time were 40-100 °C and 0.5-6 h, respectively. The central point was set at 70 °C for 3.25 h, close to the extraction conditions usually for antioxidant herbal phytocompounds. After extraction, sample was cooled to room temperature and centrifuged (10000 rpm, 15 min). The supernatant was collected, concentrated in vacuo, and freeze-dried. Extract yield (% w/w) was calculated as the percentage of freeze-dried product to Chlorella mass on dry basis.
|
Independent Variable |
Coded |
Levels |
||||
|
-1.414 |
-1 |
0 |
1 |
1.414 |
||
|
Temperature (°C) |
X1 |
40 |
48.8 |
70 |
91.2 |
100 |
|
Time (h) |
X2 |
0.5 |
1.3 |
3.25 |
5.19 |
6 |
Table 1 Five levels of two independent variables for extraction of antioxidant C. sorokiniana ingredients
Solubility in 75% ethanol aqueous solution
The obtained extract was dispersed in 75% ethanol aqueous solution (10-15 mg/mL, resembling the solvation conditions in the following antioxidant activity measurements) and stirred gently at ambient temperature for 1 h, following by centrifugation (10000 rpm, 15 min). After removing the supernatant, the insoluble sediment was completely dried in 105 °C and weighted. 75%-Ethanol solubility (% w/w) of water extract (termed as WS-E75S%) was calculated as the result of (100%-sediment%) on dry extract basis.
Measurement of total phenolic content (TPC)
Sample was dissolved in 10 mL deionized water (5.0 mg/mL) by heating to 100 °C for 10 min and cooled to room temperature for total phenolic content measurement, according to the method of Sato et al.18 Gallic acid in 50% ethanol aqueous solution (3-100 mg/mL) was used as reference. A portion (400 mL) of the extract solution or reference was mixed with 400 mL of Ciocalteu’s phenol reagent for 3 min, followed by successively adding with 40 mL of 10% Na2CO3 aqueous solution and stirring at every 10 min. After reaction for one hour, sample mixture was detected for the absorbance at 735 nm in a spectrophotometer (Hitachi U-2001, Tokyo, Japan). Data were calculated according to gallic acid standard curve and presented as gallic acid equivalent (GAE) mg/g extract. Three replicated measurements were done.
Measurement of total protein content
Total protein content was measured according to the method of Lowry et al.19 Sample (60 mg) was dissolved in 10 mL deionized water by heating, cooled to room temperature, and diluted to 0.6 mg/mL before reaction with Lowry reagent and Folin-phenol reagent at room temperature for 45 min. The absorbance at 540 nm was examined and calibrated with bovine serum albumin (BSA) standard curve. Data were means of three replications.
Measurement of total carbohydrate content
Total carbohydrate content was examined by using the phenol-sulfuric acid method20 with absorbance at 488 nm as an index and glucose as standard. Data were means of three replications.
Characterization of phenolic compounds profile
75%-Ethanol soluble of Chlorella water extract (WS-E75S) was examined by high performance liquid chromatography with a photo di array detector (HPLC-DAD) and Polaris C18 column (5m, 250 x 46 mm, Varian). Sample was filtered through 0.45 mm membrane before HPLC analysis. The elution condition was: acetonitrile/0.1% phosphoric acid, flow rate: 1 mL/min, at ambient temperature. Phenolic compounds including ChA, ECG, EGCG, GA, and GCG were used as standards.
Analysis of neutral monosaccharide compositions
Sample was subjected to hydrolysis in 2 M trifluoroacetic acid (5 mg/mL) in a boiling water bath for 6 h, followed by vacuum drying in a centrifugal evaporator (Savant Speed-Vac model 100 evaporator, Savant Instruments, Inc. NY). The hydrolysate was re-dissolved in 10 mL of de-ionized water (18 MW) and ion exchanged on Amberlite IRA-400 resins (Cl- form) to remove acidic residue completely. Sample was then filtered through a 0.45 mm membrane for analysis by high performance anion exchange chromatography (HPAEC), using a Dionex DX-500 equipped with an ED40 detector and CarboPacTM PA1 guard (50x4 mm ID) and analysis (250x4 mm ID) columns (Dionex Co., Sunnyvale, USA). The electric pulses of ED40 detector was set as: output range: 100 nA; E1: +0.05 V, t1: 0.00-0.40 s; E2: +0.75 V, t2: 0.41=0.60 s; E3: -0.15 V, t3: 0.61-1.00 s. The elution condition for analysis was 4 mM NaOH at 0.75 mL/min and at ambient temperature. Sampling size was 100 mL. Time for data collection was 30 min after injection. Chromatograms were analysed with the Dionex Peak Net System (Dionex Co.). Data were obtained by calibration with monosaccharide standard curves: A: 9.00´105×C-8.03´104 (R2: 0.995) for arabinose; A: 5.66´105×C+2.24´106 (R2: 0.984) for fucose; A: 6.59´105×C+2.48´106 (R2: 0.995) for glucose; A: 9.42´105×C-1.48´106 (R2: 0.998) for galactose; A: 9.72´105×C-4.40´106 (R2: 0.992) for mannose; A: 7.48´105×C-2.75´106 (R2: 0.995) for rhamnose; A: 9.34´105×C=4.49´106 (R2: 0.989) for ribose; and A: 1.03´106×C-3.81´106 (R2: 0.995) for xylose; where A: peak area, C: monosaccharide concentration (mg/mL). All data were measured in three replications and presented as molar percentage (mol%) on total neutral monosaccharide basis.
Molecular distribution analysis
Sample (2 mg/mL) was dissolved in deionized water by heating at 95 °C for 2 h, followed by cooling and pre-filtering through 0.45 mm before measurement by high-performance size exclusion chromatography (HPSEC). According to the analysis conditions in our previous report,21 a high-resolution differential RI detector (Viscotek Co., Texas, USA) and guarded TSK GMPWXL column were employed. Elution was done with 50 mM NaNO3 aqueous solution (containing 0.02 % (w/w) NaN3) at a flow rate of 0.5 mL/min at 35 °C . Chromatograms were collected in triplicate. Data were managed with TriSEC GPC software (Viscotek, Co.) and calibrated with pullulans standards (Shodex Co. Ltd., Kawasaki, Japan).
DPPH Radical scavenging ability assay
The assay was done according to the method of Shimada et al.22 a, a-Diphenyl-b-picrylhydrazyl radicals (DPPH×) at 10 mM was freshly prepared in methanol in a brown bottle before use. Sample was well dissolved in 75% ethanol solution as mentioned above. One mL of sample (1-20 mg/mL) in 75% ethanol solution was mixed with 0.25 mL of 10 mM DPPH× solution, followed by stirring for mixing well and staying in the dark at ambient temperature for 30 min. The mixture was immediately examined on the absorbance at 517 nm (A517) in a spectrophotometer (Hitachi U-2001, Hitachi Instruments, Inc., Japan). DPPH-radical scavenging effect (%) was calculated as Eq. (1). All data were measured in three replications.
Chelating ability assay on Fe2+ ions
The method of Dinis et al.23 was applied. Sample was dissolved in 75% ethanol aqueous solution as mentioned above. A portion (0.2 mL) of sample solution (1-30 mg/mL) was added with 0.74 mL methanol and 0.02 mL of 2 mM FeCl3 aqueous solution (in deionized water) for 30 sec, followed by immediately adding with 0.04 mL of 5 mM Ferrozine (in deionized water), mixing well, staying for 10 min in the dark at ambient temperature, and soon measured on the absorbance at 562 nm (A562). Chelating effect on Fe2+ was calculated as Eq. (2). All data were measured in three replications.
Reducing power assay
The reducing power assay was done according to the method of Yen et al.24 Sample was dissolved in 75% ethanol solution as mentioned above. A portion (150 mL) of sample (0.039-5.0 mg/mL) was mixed well with 150 mL of 0.2 M sodium phosphate buffer (pH 6.6) and 150 mL of 1% (w/w) potassium ferricyanide (K3Fe(CN)6) (in deionized water), following by reaction at 50 °C in a water bath for 20 min, cooling in an ice bath for 3 min, and immediately adding with 150 mL of 10% (w/w) trichloroacetic acid (in deionized water) to stop reaction. The mixture was then added with 0.6 mL deionized water and 120 mL of 0.1% (w/w) FeCl3 (in deionized water), mixed well, stayed at room temperature for 14 min, and immediately detected on the absorbance at 700 nm (A700). All data were measured in three replications. The higher the A700, the greater is the reducing power.
Statistical analysis
A RSREG program and canonical analysis of SAS software 8.12 (SAS Institute, Inc., Cary, USA) was applied to give predicted polynomials. According to the polynomials, counter plots were produced with Sigma Plot 8.0 software (Systat software Inc., CA, USA). Person’s correlation analysis was done with SAS software 8.12. General mathematic treatments for data and calibrations were carried out with Excel 2012 (Microsoft Co., USA).
Yield, 75% ethanol solubility, and total phenolic content
Table 2 illustrates that C. sorokiniana water extracts showed a yield in the range of 16.2-18.0% (w/w) on Chlorella biomass basis, WS-E75S% (75% ethanol solubility, in the same solvent for antioxidant activities assay) in the range of 30.7-38.0% (w/w) on extract basis, and total phenolic content (TPC) in the range of 2.62-3.17 GAE mg/g on extract basis. Generally, high extraction temperatures (91.2 or 100 °C) tended to give high values in yield, WS-E75S%, or TPC. The extracts obtained under the repeated central condition (70 °C, 3.25 h; trials #9-14) exhibited a comparatively low yield (16.6-17.1% w/w), low WS-E75S% (30.7-34.0% (w/w)), and intermediate TPC (2.84-3.08% w/w) among the samples studied. For those extracted at 70 °C, prolonging extraction time to 6 h (trial #8, 70 °C, 6 h) resulted in a reduced TPC (2.62 GAE mg/mL), as compared with those extracted for 0.5 or 3.25 h (trials #7, 9-14). The maximal yields in this study agree closely with that (18%) of 80 °C water extract from the same Chlorella source.25
|
Trial No |
Extraction Condition |
Yield1 |
WS-E75S2 |
Total Phenolic Content3 |
|
|
Coded |
Practical |
(% w/w) |
(% w/w) |
(GAE mg/g) |
|
|
1 |
-1,-1 |
48.8 °C, 1.3 h |
16.3±1.04 |
34.3±4.5 |
2.83±0.02 |
|
2 |
+1, -1 |
91.2 °C, 1.3 h |
18.0±0.4 |
34.4±4.3 |
2.73±0.06 |
|
3 |
-1, +1 |
48.8 °C, 5.19 h |
16.6±0.2 |
37.6±2.3 |
3.04±0.03 |
|
4 |
+1, +1 |
91.2 °C, 5.19 h |
17.4±0.2 |
35.9±4.5 |
3.17±0.07 |
|
5 |
-1.414, |
40 °C, 3.25 h |
16.6±0.6 |
35.7±1.7 |
2.82±0.07 |
|
6 |
+1.414, 0 |
100 °C, 3.25 h |
17.9±0.9 |
38.0±3.6 |
2.87±0.09 |
|
7 |
0, -1.414 |
70 °C, 0.5 h |
16.2±0.1 |
32.0±3.4 |
2.99±0.11 |
|
8 |
0, +1.414 |
70 °C, 6 h |
17.0±0.2 |
34.9±4.5 |
2.62±0.08 |
|
9 |
0, 0 |
70 °C, 3.25 h |
16.7±0.3 |
31.9±1.6 |
3.03±0.09 |
|
10 |
0, 0 |
70 °C, 3.25 h |
16.9±0.6 |
30.7±1.4 |
2.84±0.14 |
|
11 |
0, 0 |
70 °C, 3.25 h |
16.6±0.3 |
34.0±3.8 |
2.91±0.32 |
|
12 |
0, 0 |
70 °C, 3.25 h |
17.1±0.6 |
33.3±3.1 |
2.92±0.15 |
|
13 |
0, 0 |
70 °C, 3.25 h |
16.7±0.7 |
31.6±1.3 |
3.08±0.09 |
|
14 |
0, 0 |
70 °C, 3.25 h |
17.1±0.4 |
32.6±2.2 |
2.90±0.06 |
Table 2 Yields, 75% ethanol solubility, and total phenol contents of C. sorokiniana water extracts under 14 extraction conditions designed with 2-factor, 5-level central composite experimental design
1On dry Chlorella biomass basis.
2WS-E75S%: 75% ethanol solubility of water extract, on dry extract basis.
3GAE: Gallic acid equivalent on dry extract basis.
4Means ± standard deviations (n = 3).
Antioxidant activities
Figure 1 depicts the concentration dependencies of the antioxidant parameters examined for Chlorella water extracts. Generally, the concentration-induced increments in DPPH× scavenging extent were found the greatest for trials #2 and 7 and least for #8-14 (Figure 1A). Those in Fe2+ chelating extent were the greatest for #2 and 6 and least for #1, 3, and 9-14 (Figure 1B). And, those in reducing powers (indicated by A700) were the greatest for #5 and 6 and least for #8-14 (Figure 1C). Table 3 illustrates that the maximal DPPH× scavenging extent was observed in the range of in average 79.0-95.1% at the extract concentration studied (10-20 mg/mL). By interpolating data curves at 50% extent in Figure 1A, 50% DPPH×-scavenging concentration (SC50) was found in the range of 3.85-7.36 mg/mL on extract basis (equivalently 1.37-2.40 mg/mL on WS-E75S basis). The maximal Fe2+ chelating extent was in the range of 55.1-80.9% in average, accompanying with a 50% Fe2+ chelating concentration (CC50, obtained from Figure 1B) in the range of 10.4-23.8 mg/mL on extract basis (equivalently 3.95-8.95 mg/mL on WS-E75S basis). As to reducing power, the concentration (C)-induced increments (A700/C) ranged from 0.028 to 0.044 mL/mg. The lowest DPPH× scavenging SC50 values (1.37 mg/mL on WS-E75S basis, the same solvent as for DPPH× scavenging assay) were about double of that of antioxidative peptide purified from C. ellipsoidea protein (Leu-Asn-Gly-Asp-Val-Trp, 50% scavenging concentration: 0.92 mM, i.e. 0.646 mg/mL).15

Figure 1 Depicts the concentration dependencies of the antioxidant parameters.
Changes in DPPH× scavenging extent. B. Fe2+ chelating extent. C. Reducing power of C. sorokiniana water extracts with extract concentration (extraction conditions as indicated in Table 2, where ○ = #1, ●= #2, = #3, ▲= #4, ▽ = #5, ▼= #6, □= #7, ▓= #8, ◇= mean of #9-14).
|
Trial No |
Extraction Condition |
DPPH × Scavenging Ability |
Fe2+-Chelating Ability |
Reducing Power C-Dependence |
||
|
Maximum |
SC501 (mg/mL) |
Maximum |
CC502 (mg/mL) |
A700/C3 (mL/mg) |
||
|
1 |
48.8 °C, 1.3 h |
84.5±0.84 |
4.75 |
56.1±2.54 |
22 |
0.041 |
|
2 |
91.2 °C, 1.3 h |
95.1±1.0 |
4.35 |
76.1±0.7 |
12.8 |
0.042 |
|
3 |
48.8 oC, 5.19 h |
79.0±0.5 |
4.85 |
55.1±1.7 |
23.8 |
0.036 |
|
4 |
91.2 °C, 5.19 h |
90.9±0.1 |
4.73 |
73.4±1.4 |
13.3 |
0.036 |
|
5 |
40 °C, 3.25 h |
90.6±0.1 |
3.85 |
60.0±3.4 |
18.2 |
0.043 |
|
6 |
100 °C, 3.25 h |
91.2±0.3 |
4.55 |
80.9±0.7 |
10.4 |
0.044 |
|
7 |
70 °C, 0.5 h |
90.9±0.4 |
4.35 |
72.9±2.3 |
15 |
0.039 |
|
8 |
70 °C, 6 h |
93.8±0.1 |
6.9 |
69.4±1.3 |
18.6 |
0.031 |
|
9 |
70 °C, 3.25 h |
94.8±0.1 |
5.72 |
57.3±1.3 |
15 |
0.0337 |
|
10 |
70 °C, 3.25 h |
94.9±0.0 |
6.38 |
55.8±0.9 |
15.5 |
0.034 |
|
11 |
70 °C, 3.25 h |
94.7±0.1 |
6.4 |
60.5±1.4 |
15.8 |
0.031 |
|
12 |
70 °C, 3.25 h |
87.5±0.5 |
6.5 |
58.7±2.1 |
16 |
0.03 |
|
13 |
70 °C, 3.25 h |
92.4±0.4 |
7.15 |
66.5±1.7 |
18 |
0.031 |
|
14 |
70 °C, 3.25 h |
89.4±0.1 |
7.36 |
64.4±2.0 |
19.1 |
0.028 |
Table 3 DPPH× Scavenging ability, Fe2+ chelating abilities, and reducing power concentration dependence of C. sorokiniana water extracts
1SC50: 50%-DPPH× scavenging concentration on the dry basis of extract.
2CC50: 50%-Fe2+ chelating concentration on the dry basis of extract.
3Calculated from the data at C = 15 mg/mL.
4Means ± standard deviations (n = 3).
Interactive influences of extraction temperature and time
All data in Table 2 & Table 3 were statistically analyzed by RSREG program of SAS software. The estimated polynomials for yield, WS-E75S%, SC50 on DPPH× radicals and CC50 on Fe2+ ions, except TPC and reducing power, showed sufficiently high multiple correlation coefficients (R2: 0.789-0.822). From Table 4, it is indicated that yield (% w/w, on dry biomass basis): 16.2 + 0.525 T-0.0323 t - 0.00479 T×t-0.0211 T2 + 0.000516 t2*. Prolonging long time (t2 term) tended to increase yield significantly (P < 0.05). WS-E75S% (w/w, on dry extract basis): 55.3-0.695 T**+0.0953t-0.00966 T×t+0.00525 T2***+ 0.176 t2. For scavenging DPPH×, SC50 (mg/mL): -9.22+0.387 T**+1.07 t+0.00171 T×t-0.00278T2***-0.143 t2. Both WS-E75S% and SC50 values varied with T and T2 significantly (P < 0.01 and P < 0.001). And, for chelating Fe2+ ions, CC50: 22.2+0.00835T- 0.,223t- 0.00781T×- 0.00117 T2*+0.192 t2, varying significantly and negatively with T2 (P < 0.05).
|
Variable |
Regression Equation1 |
R22 |
|
|
Yield (% w/w) |
16.2 + 0.525 T - 0.0323 t - 0.00479 T×t - 0.0211 T2 + 0.000516 t2* (saddle point at 58.7 °C, 5.75 h) |
0.822 |
|
|
WS-E75S (% w/w) |
55.3 - 0.695 T** + 0.0953 t -0.00966 T×t + 0.00525 T2***+ 0.176 t2 (minimal at 67.7 °C, 1.59 h) |
0.831 |
|
|
DPPH× scavenging SC50 (mg/mL) |
-9.22 + .387 T** + 1.7 tt + .171 T×t -.278 T2**- .143 t 2 (minimal at 7.8 °C, 4.17 h) |
0.818 |
|
|
Fe2+ chelating CC50 (mg/mL) |
22.2 + 0.00835 T - 0.223 t - 0.00781 T×t - 0.00117 T2*+ 0.192 t2 |
0.789 |
|
|
|
Fe2+ chelating CC50 (mg/mL) |
22.2 + 0.00835 T - 0.223 t - 0.00781 T×t -0.00117 T2*+ 0.192 t2 |
0.789 |
Table 4 Regression equations for the yield, 75% ethanol solubility (WS-E75S), 50%-DPPH× scavenging concentration (SC50), and 50%-Fe2+ chelating concentration (CC50) of C. sorokiniana water extracts in terms of extraction temperature and time
1T = temperature (°C), t = time (h); *, **, and *** = significant levels at P < 0.05, < 0.01, and < 0.001, respectively.
2R2 = multiple correlation coefficients by least-squared fits.
The counter plots corresponding to the above polynomials were produced and are shown in Figure 2, in order to illustrate the interactive effects of temperature and time concerned. When > 70 oC, T became the dominant variable governing the yield (Figure 2A), accompanying with a saddle point at 58.7 °C for 5.75 h. The WS-E75S% (Figure 2B) minimized at 67.7 °C for 1.59 h and increased mainly with increasing or reducing T away from 67.7 °C or increasing t. The SC50 (Figure 2C) maximized at 70.8 °C for 4.17 h and reduced mostly by increasing or decreasing T with shortening t. And, the CC50 reduced mainly with increasing T (Figure 2D). Based on the high yield, WS-E75S%, and antioxidant activities (i.e. low SC50 and CC50), the optimal extraction condition can be concluded at 100 °C for 1 h.
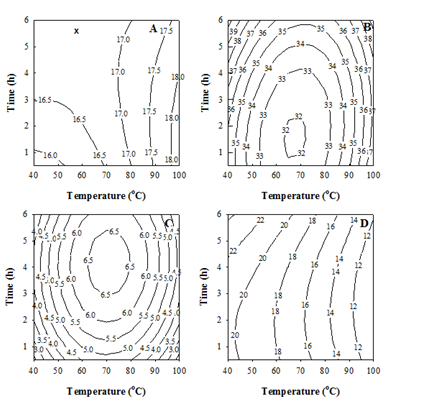
Figure 2 Counter plots for the yield. (A) WS-E75S%. (B) 50% DPPH×-scavenging concentration (SC50).
(C) 50% Fe2+-chelating concentration (CC50). (D) Chlorella sorokiniana extracts, with respect to the extraction temperature and time. Numbers indicated on counter lines: response values in the same units as in Table 2 & 3.
Characterization of 75% ethanol-soluble phytocompounds
Samples WS-E75S were concerned due to playing a key role for the antioxidant activities. All WS-E75S from 9 extracts with different extraction conditions showed similar HPLC profiles as shown in Figure 3A, exemplified with the WS-E75S from extract at 70 °C for 3.25 h. Typically, there were three major compounds (1-3) and four minor ones (4-7) implied from the chromatographic peaks. The UV spectra (Figure 3B) & (Figure 3C) and maximal wavelengths (lmax) (Table 5) indicate that all compounds showed three absorption peaks in the range of 190-320 nm, implying three types of chromophores with lmax at 196-198 nm (peak I); 210-213 (for compounds 2-4, 6) or 219-222 nm (compounds 5, 7) (peak II); and 256-259 (compounds 2-4 and 6) or 278-281 nm (compounds 5 and 7) (peak III). Compound 1 showed a similar UV spectrum (not shown) to that did compound 2. Generally, compounds 5 and 7 were of different structural features from those of compounds 1-4 and 6.
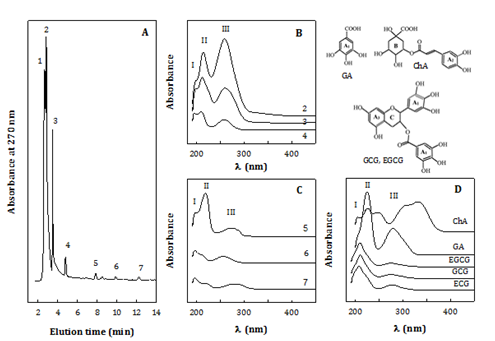
Figure 3 Characterization of 75% ethanol-soluble phytocompounds.
HPLC chromatograms. B-C) UV spectra.
D) 75% ethanol soluble (WS-E75S) from Chlorella water extract # 9 (70 °C, 3.25 h), in reference to UV-spectra of antioxidant phenolic standards.
HPLC-DAD condition: photodiarray detector; Polaris C18 column eluted with acetonitrile/0.1% phosphoric acid; flow rate: 1 mL/min. Standards ChA: Chlorogenic acid; ECG: (-) Epicatechin Gallate; EGCG: (-) Epigallocatechin Gallate; GA: Gallic Acid; and GCG: (-) Gallocatechin Gallate.
|
Peak No. |
Retention Time (min) |
lmax2 (nm) |
Standard3 |
Retention Time (min) |
lmax(nm) |
|
1, 2 |
2.62, 2.83 |
212, 256 |
GA |
4.28 |
225, 280 |
|
3 |
3.46 |
198s, 213, 259 |
ChA |
10.07 |
204, 225, 245, 303, 334 |
|
4 |
4.76 |
196s, 210, 256 |
EGCG |
10.61 |
210, 275 |
|
5 |
7.74 |
198, 219, 278 |
GCG |
12.52 |
210, 275 |
|
6 |
9.88 |
197, 210s, 256 |
ECG |
16.06 |
208, 278 |
|
7 |
12.05 |
197, 222s, 281 |
Table 5 Retention times and maximal-absorbance wavelengths (lmax) of chromatographic fractions from Chlorella WS-E75S and phenolic compound standards eluted by HPLC-DAD1
1High performance liquid chromatography with a photodiarray detector and the analysis conditions explained in Figure 3.
2The wavelength at which UV absorbance maximized for a peak.
3Phenolic standards ChA: Chlorogenic Acid; ECG: (-) Epicatechin Gallate; EGCG: (-) Epigallocatechin Gallate; GA: Gallic Acid; and GCG: (-) Gallocatechin Gallate.
For structural elucidation, small compounds possibly present in WS-E75S were focused on phenolic compounds, nucleic acids, unsaturated fatty acids such as a-linolenic acid and analogues, based on three considerations. Firstly, the spectra of the compounds in this study (Figure 3B) & (Figure 3C) were identified in reference to several typical phenolic compounds (Figure 3D & Table 5). It is indicated that GA displayed a typical UV spectrum of two big absorption peaks with lmax: 225 and 280 nm, indicating its phenolic ring (p®p* transition of C:C bonds in A1 ring) and free carboxyl group (n®p* transition of C:O bonds), respectively. EGCG, GCG, and ECG exhibited similar spectra of a big, broad peak with lmax: 208-210 nm and mild broad one with lmax: 275-278 nm, concerning C: C bonds in 3 phenolic rings (2 A1 and A3 rings) and C: O bonds in ester linkage, respectively. And, ChA showed a triplet at 190-278 nm and duplet at 278-380 nm, contributed by three kinds of C:C bonds in caffeic acid group (A2) and two types of C: O bonds attached to quinic acid group (B), respectively. Evidently, all sample compounds were different from these phenolic standards on the viewpoints of UV spectra and HPLC retention time. Secondly, WS-E75S samples are reasonably expected to contain small molecules such as nucleotides, monosaccharides, unsaturated (omega-3) fatty acids or derivatives originating from their parent extracts (C. sorokiniana and C. pyrenoidosa) with known related compositions.5,13,14,16 Thirdly, the above standard spectra and documented UV spectra13,26-30 conclude that lmax values are generally 185-200 nm for ethylene (RHC:CHR) and C: N groups; 208-225 nm for acylic dienes (phenyl, unsubstituted, conjugated or heteroannular) and buta-1, 3-diene (C:C-C:C); 250-260 nm for hexa-1, 3, 5-triene (C:C-C:C-C:C) and nucleic acids; and 270-280 nm for carboxyl groups (RRC:O) in aromatic amino acids, proteins, ketones, and fatty acids; and 300-335 nm and higher lmax values for conjugated C: O groups in esters, acetals, conjugated or condensed aromatic compounds.
Accordingly, the compounds 1-4 and 6 were likely nucleic acids (lmax: 210-220 and 260 nm) and analogues with ethylene chromophores (lmax: 196198 nm).25 The ethylene group leveled greater in the compounds eluted for a longer retention time. The compounds 5 and 7 were possibly related to W-3 fatty acids such as a-linoleic and linolenic acids (lmax: 217-219 and 270 nm),29 fatty alcohols, or phytols,30 those with a significant level of acyl dienes or buta-1, 3-diene (C:C-C:C) (lmax: 219-222 nm) (compound 5) or ethylene (compound 7) chromophore. Both had carboxyl groups likely conjugated to phenyl rings, ketones, aromatic amino acids, or nucleotides (lmax: 278-281 nm) under the bathochromic (lmax red shifting) and hyperchromic (enhanced absorptive) effects by conjugation.26
It is interesting to found that in this study the HPLC peak area of only compound 5, rather than the other compounds, correlated significantly and positively with the DPPH×scavenging ability (Person’s correlation coefficient: 0.733*, P < 0.05). This agrees with the estimation about its W-3 fatty acid compositions that are known of high radical scavenging abilities and have been found abundant in C. sorokiniana lipid extract.12 Compound 5 could be a good index responsible for the antioxidant activity in scavenging DPPH× radicals of Chlorella extracts products. As to the compounds 1-4 and 6 (lmax: 260 nm), they could be the quality index of functional drinks with CGF, namely Chlorella hot water extracts,3-5 agreeing with the absorbance at 260 nm, an old index in Chlorella industry.
For WS-E75S compounds, no UV signals about proteins (typically lmax: 230 and 280 nm),28 flavonoids (typically lmax~214, 270, and 339 nm),31 b-carotene or chlorophylls (lmax in the range of 400-680 nm) that are usually found in Chlorella biomass9 were dateable in this study.
Characterization of biopolymer compositions
According to the optimal extraction condition concluded from Figure 2, the water extract at 100 °C for 1 h (WS100) was specially prepared for further analysis on carbohydrates, proteins, and molecular property. It is shown that WS100 contained a total protein content: 23.5±0.1% (w/w) (by Lowry assay) and total carbohydrate content: 22±1% (w/w) (by phenol-sulfuric acid method). Figure 4A shows that glucose (Glc) and ribose (Rib) were dominant, followed by galactose (Gal) and rhamnose (Rha) in the neutral monosaccharides of WS100 acid hydrolysate. Mannose (Man), xylose (Xyl), and arabinose (Ara) were almost insignificant. The monosaccharide composition was Glc:Rib:Gal:Rha:Man:Xyl:Ara: 52.4:25.9:8.6:7.3:3.0:2.2:0.7 in mol%, expectedly coming from nucleic acids and biopolymers (polysaccharides and glycoproteins). It possessed two molecular fractions (Figure 4B) as 57.8% F1 fraction (Mw: 25.0±0.6 kDa) and 42.2% F2 fraction (Mw: 0.95±0.09 kDa). From the results of Figure 2, the properties of Chlorella water extract could be semi-empirically estimated as yield~18% (w/w), WS-E75S ~37% (w/w), statistically predicted DPPH× scavenging SC50~3.0 mg/mL, and Fe2+ chelating CC50~11 mg/mL.

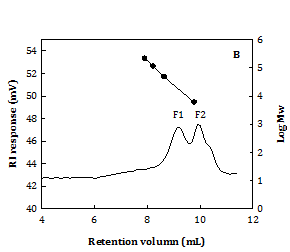
Figure 4 It shows the retention times of glucose and rhibose.
A. Neutral monosaccharide compositions by HPAEC B. Molecular distribution by HPSEC. C. Hot-water extract from C. sorokiniana (extraction condition: 100oC for 1 h). Ara: Arabinose, Gal: Galactose, Glc : Glucose, Man: Mannose, Rha: Rhamnose, and Rib: Ribose. Calibration standards: Pullulans (·).
Generally, the WS of C. sorokiniana (previously classified as C. pyrenoidosa) in this study showed a lower content in total carbohydrates (rich in Glc and Rib) or crude proteins than those did C. vulgaris.32 Glc and Rib can be linked to the presence of starches and nucleotides in Chlorella biomass.5,13 Generally, Gal and Rha are the major monosaccharides in cell wall compositions of C. sorokiniana and C. pyrenoidosa.33 And, Man and Gal are found dominant in the immunomodulatory polysaccharides or glycoproteins from C. pyrenoidosa7,14,34 and C. vulgaris.4,5 In contrast to the biopolymers in Chlorella water extracts at 100 °C for 1 h in this study, the purified polysaccharide fractions from C. pyrenoidosa water extracts at 100 °C for 4 h show a greater percentage of large molecular weight fraction (76% F1), higher Mw values (82 k and 1.7k) and rich in Man.14 Different results between different studies can be attributed to the differences in Chlorella species, cultivation conditions, extraction conditions, and isolation processes applied.
In this study, the estimated polynomials for yield, WS-E75S%, DPPH× scavenging SC50 and Fe2+ chelating CC50 as a function of temperature and time had been statistically obtained and could explain 78.9-82.2% of data variations. An optimal extraction condition was discovered at 100 °C for 1 h, associated with the highest yield (~18% (w/w)), WS-E75S% (~38% (w/w)), and antioxidant activities (statistically predicted SC50~3.0 mg/mL and CC50~11 mg/mL). The antioxidant activities could be mainly related to its phenolic compounds (TPC~3.17 GAE mg/g) and WS-E75S, and partly to biopolymers. Where the WS-E75S was mainly composed of compounds like nucleic acids and analogues with ethylene structure and polyunsaturated fatty acids, fatty alcohols, or phytols with acyl dienes, buta-1, 3-diene or ethylene structure. The water extract at 100 °C for 1 h contained neutral carbohydrates at a ratio of Glc:Rib:Gal:Rha:Man:Xyl:Ara: 52.4:25.9:8.6:7.3:3.0:2.2:0.7 in mol% and two molecular fractions. These results will facilitate developing new combined processes involving counter-current chromatography for continuously collecting functional ingredients from Chlorella water extract (CGF), such as antioxidant WS-E75S (ribose, nucleic acids, polyunsaturated fatty acids, and their derivatives) and immunomodulator biopolymers, and from the residues after water extraction. More investigations will be done in the future on examining the functionality in vitro and in vivo of the isolates and on optimal isolation processes and characterization for more functional ingredients from Chlorella residues.
This work was financially supported by National Science Council, Executive Yuan of Taiwan (NSC 94-2745-B-126-003-URD). The authors thank Taiwan Chlorella Manufacturers Ltd (Taipei, Taiwan) for kindly providing Chlorella sample.
The authors declare that there are no conflicts of interest.

©2017 Lai, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.