Journal of
eISSN: 2373-6410


Case Report Volume 7 Issue 1
1Senior Clinical Fellow Neurology, Salford Royal Foundation Trust, UK
21st Neurology Department, Aristotle University, Greece
3Department of Radiology, Aristotle University, Greece
Correspondence: Thaleia Kalatha, Senior Clinical Fellow in Neurology, Salford Royal Foundation Trust, Stott Lane, Salford, M6 8HD, England, Tel 447804910889
Received: May 20, 2017 | Published: June 19, 2017
Citation: Kalatha T, Tegos T, Papaioannou A, Charitanti-Kouridou A, Orologas A (2017) Multiple Spinal Cord Infarction due to Primary Antiphospholipid Syndrome. J Neurol Stroke 7(1): 00226. DOI: 10.15406/jnsk.2017.07.00226
Spinal cord infarction (SCI) is quite rare and is usually presented with a sudden onset paralysis, sensory disturbances, lumbar pain and cystic disorders depending on the location of the infarct. A variety of causative factors has been described, but primary antiphospholipid syndrome only in a limited number of case reports. We discuss a case of a 49year old woman with acute right hemiparesis and sensory disturbances below C7 level. T2 weighted sequence MRI showed multiple small hyper intense lesions in the right lateral spinal cord from C6 to C7 and in the poster lateral spinal cord in T1/T2 level consistent with ischemia. The laboratory work-up returned the diagnosis of primary Antiphospholipid syndrome with positive lupus anticoagulant antibodies. This is a case of primary Antiphospholipid syndrome first manifested with multiple ischemic lesions of the spinal cord.
Keywords: Antiphospholipid antibodies; Antiphospholipid syndrome; Lupus anticoagulant antibody; Spinal cord infarction
Spinal cord infarction (SCI) is considered rare in comparison with cerebral infarction but its consequences can lead to greater disability. Reliable incidence rates do not exist due to publication of case reports or cases series [1,2]. A retrospective one-center study finds spinal cord infarction accountable for 1.2% of the total admissions [3]. Etiologies of spinal infarcts include atheromas involving the aorta as the most common cause, usually after a thoraces abdominal aneurysm repair. Other causes of SCI include spinal arteriovenous malformation repair, dissecting aortic aneurysm, cardiac arrest, embolic infarction, syphilitic angiitis, and less common etiologies include collagen disease as systemic lupus or polyarteritis nodosa, pregnancy, sickle cell disease and neurotoxic effects of iodinated contrast material [4]. Syphilitic arteritis was the most frequent cause of SCI until the introduction of penicillin [5]. Neurovascular syndromes of SCI have different symptomatology depending on the level of the infarction and arteries involved, but they usually present with weakness, radicular back pain, are flexia, spin thalamic sensory loss, and autonomic dysfunction.
Primary Antiphospholipid syndrome is quite rare as an etiologic factor for SCI. One case of recurrent anterior spinal artery syndrome has been described with positive aCL [6] and another case of childhood Antiphospholipid syndrome [7]. Stroke and TIA, both cerebral and spinal, accounted for 22.9% of the initial manifestations of Antiphospholipid syndrome in a patient cohort of 1000 patients (Euro-Phospholipids Project Group study) [8].
SCI is usually affecting anterior spinal artery due to the difference in collateral supply between the anterior and posterior spinal artery, so SCIs involving the posterior artery supply are considered rare in comparison to the ones involving anterior spinal artery. Anterior spinal artery syndrome is a clinical entity occurring due to infarction in the perfusion territory of anterior spinal artery with well-described consequences. Also, spinal cord infarctions restricted to a unilateral territory have been very rarely described to our knowledge [9]. We therefore report the findings of a patient, whose symptomatology results from multiple infarctions involving different perfusion territories; the unilateral perfusion territory of the anterior spinal artery, presumably that of a sulco-commissural artery, the ipsilateral peripheral vasocorona as well as the ipsilateral posterior spinal artery.
A 49-year-old woman was admitted in the 1st Neurology clinic of Aristotle university of Thessaloniki after an acute onset of right hemiparesis, numbness of her left side and parenthesis on her right side below C7 level. She had been experiencing parenthesis on her right hand over the last twelve hours. The next several hours, she developed urinary retention followed by deterioration of numbness and weakness in the upper and lower extremities. She complained of severe back pain. Neurological examination revealed normal speech, cognition, and a mild right Horner’s syndrome. Motor examination on the left was normal. On the right side she had paresis of the right upper extremity with wrist extension 4/5, grip strength 3/5 and thumb abduction 3/5. Hip flexion on the right lower extremity was 2/5; hip extension 2/5;hip adduction and abduction 2/5; knee extension 3/5; knee flexion 3/5 right, ankle dorsifexion 3/5; ankle plantar flexion 3/5 and foot eversion and first-toe extension 3/5.There wasn’t any spasticity noted. Reflexes were present bilaterally in the biceps, triceps, brachioradialis, while patellar and Achilles jerks were mild. Plantar responses were normal. Rectal sensation was intact. Pain and temperature sensations were impaired below the C7 dermatome with pareasthesia on the right side and hypoesthesia on the left. Proprioception and vibration senses were intact. Hypercholesterolemia and smoking were risk factors for arteriosclerosis.
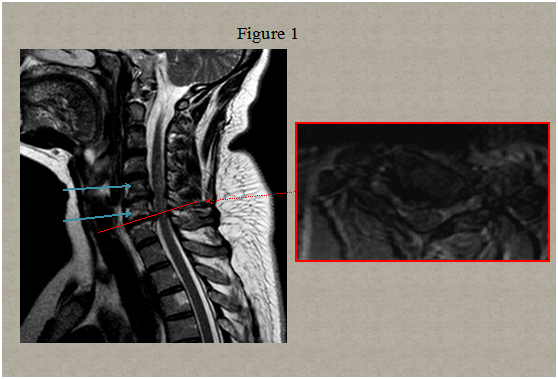
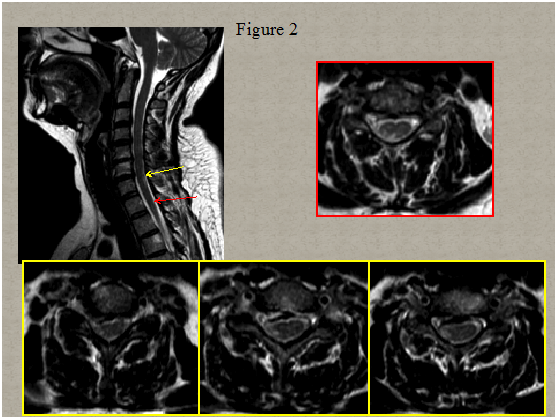
Immediate magnetic resonance imaging (MRI) of the spine was performed that showed degenerative changes with a herniated disc present at C5-C6 and C6-C7 level pressuring the spinal sac and cord and a centrolateral right disc hernia without pressure on the cord Figure 1. Two days after clinical onset electrical stimulation of the median, ulnar and peroneal nerves showed normal excitability of the right hand and leg muscles. Somato sensory evoked potentials of upper and lower limbs were normal. A second spinal MRI scan 5 days later revealed multiple abnormal T2 signals in the right lateral spinal cord from C6 to C7 and in the posterolateral spinal cord in T1/T2 Figure 2. Computed tomography angiogram of the chest, abdomen, and pelvis showed no dissection of the aorta. Tran esophageal echocardiography to evaluate for cardiac embolic source was normal. Cervical ultra sonogram showed no arteriosclerotic lesions.
The laboratory data showed positive lupus anticoagulant (LA) antibodies, while antinuclear antibodies were negative as well asanticardiolipin antibodies and b2GPI antibodies. The erythrocyte sedimentation rate was 19mm in 1hour (normal values 20mm/h). Tests for factor VIII, antithrombin III, and protein C levels, fibrin degradation products, FTA-Abs, anti DNA, and complement studies were normal. Protein S was mildly low (56, normal 60-140) as expected. Analysis of cerebrospinal fluid obtained via lumbar puncture revealed normal results.
The patient was discharged with prophylactic anticoagulant treatment (acenocoumarol) and statin therapy. The 3-month follow up revealed a persistence of the dissociated hemisyndrome but significant improvement of the motor functions. According to the 2006 criteria [10] the diagnosis of primary Antiphospholipid syndrome was made from the symptomatology, and the positive lupus anticoagulant test both in the admission and in the follow-up period (>12 weeks apart). The patient has given signed informed consent.
The radiculomedullary artery may divide into an anterior and a posterior radicular artery, but usually there is only one branch accompanying the dorsal or the ventral nerve root. Three types of radicular arteries are described: the ones that end with the nerve root at the level of dura matter not supplying the spinal cord with blood, the ones that do not enter the surrounding arterial system of the spinal cord (vasocorona) and the ones that feed the spinal cord vascular system. The number and location of the posterior radicular arteries is different than the anterior radicular arteries and only in a few segments of the spinal cord there are the both of them present at the same level.
The anterior spinal artery arises from branches of the intracranial vertebral arteries in the upper cervical region and runs along the entire length of the cord. In the cervical region, many collateral vessels from the vertebral and the sub clavian arteries meet its course. In the thoracic region, the anterior spinal artery receives few branches from the thoracic aorta resulting in sparser blood supply. So, the mid thoracic spinal region is considered the most vulnerable to vascular insufficiency, most likely in the T4 to T9 levels.
The anterior spinal arterysupplies blood in the anterior two thirds and the central region of the spinal cord through perforating vessels known as the sulco-commissural arteries (sulcal spinal arteries). These arteries branch off from the anterior spinal artery and run transversely through the median fissure, from which they enter the parenchyma. Each sulco-commissural arterysupplies one half of the spinal cord. The two posterior spinal arteries provide for the dorsal columns of the cord and the posterior horns. There are penetrating branches from the pial arterial plexus that supply the periphery and the posterior segment of the spinal cord. A watershed zone of increased ischemic vulnerability is located at the interface of the anterior and posterior spinal artery territories. Partial syndromes or atypical presentations of anterior spinal artery syndrome may be attributed to these border zones [5]. We present a case with a hemi-syndrome due to multiple infarcts concerning the sulcal spinal artery supply, as well as the ipsilateral peripheral vasocorona and posterior spinal artery in two different sites. The question upon the cause of the infarcts multiplicity and location arises. In some people there could be a duplicated part of anterior spinal artery for some distance. Therefore ischemia in the perfusion territory of a duplicated artery could provoke a unilateral infarction, as well as an incomplete linking of the posterior systems. Our patient had intact proprioception, albeit the small infarct in the right posterior column. The most inner part of the dorsal columns remained unaffected, which would be a prerequisite for a proprioception disorder.
The lack of abnormalities in the early MRI is not un proceeded and on the contrary provides support in SCI diagnosis. The other conditions that cause myelitis usually reveal abnormalities acutely in close relation to the onset of symptoms, i.e. multiple sclerosis. Angiography has not been performed in this case because of the technique involved risks as well as the evidence in favour of the diagnosis of the primary Antiphospholipid syndrome that is known to cause small vessel thrombosis and can therefore are in keeping with the findings.
The underlying mechanism can be related either to ischemic damage of neural tissue due to endothelial damage or to direct neural tissue damage by circulating antibodies. Antiphospholipid antibodies can activate platelets or endothelial cells through C activation or by binding to phospholipids or 2GPI of their membranes and thus induce a prothrombotic state [11]. Also, they produce pro-inflammatory signals. This pro-inflammatory and pro-agulant state creates microvessel thrombi and later local ischemia of the neural tissue and opening of the blood-brain barrier [12]. There have been observations upon the interaction of the antibodies with the neural tissue. Interleukin-6 release damages neuronal and astrocyte cells, while Antiphospholipid binding to neural tissue moderates its function and inhibits astrocyte proliferation in vitro [13].
Spinal cord infarction is an emergency, especially to exclude ongoing vascular aetiology (aortic aneyrysm) but once the differential diagnosis is narrowed to ischemic etiology, vascular risk factors should be examined, including the various causes of arteritis. Acute management of SCI includes close monitoring and avoidance of hypotension. After identification of a risk factor, measures to reduce the significance of the insult with appropriate medical management are undertaken. No studies are available that address specific anticoagulation measures for spinal infarction. When encountering an infarct related with Antiphospholipid syndrome, the treatment of choice according to the American Heart Association/American stroke association guidelines is long-term anticoagulation treatment [14]. The addition of further ant platelet therapy does not reduce the danger of relapse and on the contrary increases the possibility of hemorrhage. Statins also seem to help, possibly due to reducing the activation of endothelial cells and due to the inhibition of the anti-b2 antibodies related tissue factor over expression [15].

©2017 Kalatha, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.