Journal of
eISSN: 2373-4310


Review Article Volume 2 Isuse 6
Department of Health, Baylor University, USA
Correspondence: Darryn Willoughby, Ph.D, Baylor University, Department of Health, Human Performance, and Recreation, Waco, TX 76798, USA, Tel 254-710-3504
Received: September 20, 2015 | Published: October 12, 2015
Citation: McKinley-Barnard S, Willoughby DS. The potential cytoprotective influence of estradiol and fish oil supplementation on indices of exercise-induced muscle damage in females. J Nutr Health Food Eng. 2015;2(6):205-219. DOI: 10.15406/jnhfe.2015.02.00082
Exercise-induced muscle damage (EIMD) occurs following unaccustomed exercise, usually involving eccentric muscle contractions. Eccentric exercise contractions may cause harsh morphological changes in the individual muscle fiber. Various interventions have been proposed to attenuate EIMD and DOMS. An intriguing proposed intervention involves 17-β estradiol (estrogen) as an anti-oxidant. Estrogen has a cyto-protective effect on the sarcolemma, which protects the muscle from oxidative-induced muscle damage known to occur with strenuous exercise. It has been theorized that estrogen has the functional capacity to act as a membrane stabilizer, Due to the supposed cytoprotective effects of 17-β estradiol (aka. estrogen), females are thought to be less predisposed to exercise-induced muscle damage than males. However, females may be more prone to muscle damage during the low estrogen point in their 28-35day cycle (follicular phase) compared to their high estrogen point (luteal phase). Numerous treatments have been proposed to minimize muscle damage and alleviate the symptoms of DOMS, but a clear beneficial treatment has not yet been identified. Another intriguing idea is that the anti-inflammatory and anti-oxidative properties of omega-3 (n-3) fatty acids may help counteract the inflammatory state associated with muscle damage and DOMS. The omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are of interest as they are common components of fish oil supplements and have been shown to be beneficial in improving some inflammatory conditions. Therefore, fish oil supplementation has been suggested to be important for cytoprotection due to its anti-oxidant potential for significantly decreasing markers of muscle damage. Therefore, fish oil supplementation may reduce oxidative stress, thereby augmenting cytoprotection throughout the course of the 28day menstrual cycle.
Keywords: eccentric exercise, muscle damage, estradiol, female, omega-3 fatty acids
EIMD, exercise-induced muscle damage; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; DOMS, delayed onset muscle soreness; HMB, β-hydroxyl-β-methylbutyrate; TNF, tumor necrosis factor; ROS, reactive oxygen species; EC, excitation-contraction; MAPK, mitogen-activated protein kinase; H2O2, hydrogen peroxide; NO, nitric oxide; FOXO, forkhead box; (Murf1, murine ring-finger-1; NIK, NF-κb inducing kinase; IKK, iκb kinase; ROS, reactive oxygen species; SOD, superoxide dismutase; PFA, polyunsaturated fatty acids; LA, linoleic acid; COX, cyclooxygenase; LOX, lipoxygenase; PG, prostaglandins; TX, thromboxanes; LT, leukotrienes; HETE, hydroxyeicosatetraenoic acids; PPAR, peroxisome proliferator-activated receptor; CRP, c-reactive protein; MDA, malondialdehyde; TBARS, thiobarbituric acid-reactive substance
It is well known that exercise-induced muscle damage (EIMD) occurs following unaccustomed exercise, usually involving eccentric muscle contractions. An eccentric muscle contraction occurs when an external force is greater than that being exerted by the working muscle, which results in lengthening of the muscle fiber.1 During an eccentric muscle contraction, the muscle lengthens while it is trying to contract. There is a greater mechanical strain per muscle fiber with eccentric muscle contractions compared to concentric muscle contractions because fewer muscle fibers are recruited during these contractions.2 During concentric muscle contractions, the muscle is doing the work, but during eccentric muscle contractions, work is done on the muscle by the external forces.3 The ability of the muscle to resist force is approximately 30% higher during a maximal voluntary contraction compared to the muscle’s ability to exert force during a concentric contraction.4 However, while the muscle’s ability to resist force is considerably greater during eccentric contractions, the metabolic cost and neural activation is smaller compared to concentric contractions.2,5
Eccentric exercise contractions may cause harsh morphological changes in the individual muscle fiber.6 During concentric muscle contractions, the myosin cross-bridges make repeated, constant connections with actin filaments for the duration of the muscle contraction. However, eccentric muscle contractions cause the actin filaments to be pulled in opposite directions by the external forces acting on the working muscle fibers as opposed to the center of the myosin filament as with concentric muscle contractions.6
Numerous studies have determined the symptoms of EIMD, some of which include soreness,3,7 decrease in range of motion of affected limb,3,8 decrease in muscular strength,3,9 leakage of myofiber proteins into the blood, particularly creatine kinase,3,10,11 and structural damage and inflammation.3 The most frequently reported symptom of EIMD is delayed onset muscle soreness (DOMS).12 Usually, DOMS is associated with a feeling of discomfort that accompanies EIMD. Within the first 24hours following exercise, the intensity of DOMS increases, then peaks between 24 and 72hours, and finally subsides completely between 5 and 7days following cessation of exercise.13 For elite athletes, EIMD and DOMS often occur due to a sudden increase in volume or intensity of their workout, while for sedentary individuals, a single bout of eccentric exercise may result in EIMD and DOMS.14
While it has been shown that eccentric exercise is potentially damaging, it has also been shown to be beneficial to the overall gains of the muscle. Eccentric exercise has been shown to produce greater hypertrophy gains than concentric contractions,15‒19 increase eccentric contraction-specific strength16,20 (Figure 1), and eccentric contractions occur at a lower metabolic cost, resulting in a smaller stress on the cardiovascular system compared to concentric contractions.21,22 These benefits of eccentric exercise have allowed for numerous studies to advocate the importance of including eccentric exercise in training to maximize gains in hypertrophy and strength. However, the damaging effects of eccentric exercise, which are thought to be necessary for adaptive muscle remodelling,23,24 can affect later exercise sessions due to the symptoms EIMD can cause. As a result, recent research has begun looking into interventions that may alleviate negative symptoms of EIMD, particularly DOMS.
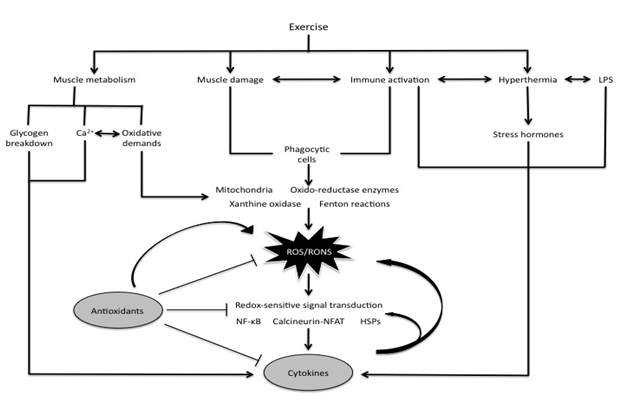
Figure 1 Interactions between exercise, ROS/RONS, antioxidants, and cytokines.
Exercise causes mechanical and metabolic stresses on skeletal muscle, thereby inducing an oxidative and inflammatory response. As a result, ROS accumulation occurs and activates NF-κB, in addition to calcineurin-nuclear T factor of activated T cells (NFAT) signaling and heat shock proteins (HSPs). Modified from Peake et al.20
Various interventions have been proposed to attenuate EIMD and DOMS, including cryotherapy, stretching, anti-inflammatory drugs, massage, exercise, β-Hydroxyl-β-Methylbutyrate (HMB), carbohydrates and proteins, and anti-oxidants. The most intriguing of these proposed interventions involves 17-β estradiol (estrogen) as an anti-oxidant. Estrogen has a cyto-protective effect on the sarcolemma, which protects the muscle from oxidative-induced muscle damage known to occur with strenuous exercise. It has been theorized that estrogen has the functional capacity to act as a membrane stabilizer, which attenuates creatine kinase and myoglobin release from muscle cells following exercise.25
Numerous treatments have been proposed to minimize muscle damage and alleviate the symptoms of DOMS, but a clear beneficial treatment has not yet been identified. Another intriguing idea is that the anti-inflammatory and anti-oxidative properties of omega-3 (n-3) fatty acids may help counteract the inflammatory state associated with muscle damage and DOMS.26 The omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are of interest as they are common components of fish oil supplements and have been shown to be beneficial in improving some inflammatory conditions.27
Circulating tumor necrosis factor-alpha (TNF-α) is a marker of acute and systemic stress-induced inflammation and has been previously shown to be released in response to eccentric exercise.28‒30 Upon its release, TNF-α binds to its trans-membrane receptor, thereby up-regulating the nuclear factor-kappa b (NF-κB) signaling cascade. The signaling pathway is responsible for up-regulating proteolysis and apoptosis within skeletal muscle.31 Therefore, being able to attenuate this cascade with fish oil supplementation could play a beneficial role in minimizing muscle proteolytic activity that has been previously shown to accompany exercise-induced muscle damage.32,33
To date, there is a paucity of studies that have examined the relationship between fish oil supplementation, DOMS, and the associated inflammatory response. Furthermore, in regards to the potential cyto-protective effects in females, no published data appear to exist. The studies that do exist typically employ a muscle-damaging workout followed by subjective measurements of pain and inflammatory responses during the following days while using older, untrained individuals. The results of these studies have been mixed. Some of the studies have shown fish oil supplementation to be effective at relieving the perceived pain associated with DOMS after exercise,34,35 while others have shown no effect of fish oil on inflammatory markers and DOMS.36‒38 Additionally, the extent of muscle damage contains individual variability and may be potentially explained by factors including the exercise type and intensity, fitness level, age, and gender.25 However, the limited number of studies and mixed results warrants further investigation into the possible benefits of fish oil on markers of muscle damage in females, particularly during periods of low and high estradiol levels.
The exact mechanisms of EIMD are not exactly known, but Armstrong39 has proposed four stages of muscle injury that are still widely accepted: initial events, autogenic processes, phagocytic processes, and regenerative phase.
Initial phase
The initial events of muscle damage are thought to occur either by mechanical or metabolic stressors,39 and may occur simultaneously.12 Increased tension, or an imbalance in tension that is associated with eccentric contractions may disrupt the sarcolemma, the sarcoplasmic reticulum (both of which cause a disruption in skeletal muscle calcium homeostasis), and myofibrillar structures.39 During repeated eccentric contractions it is proposed that the number of disrupted sarcomeres increases until membrane damage occurs (Figure 2).40 Once membrane damage occurs, damage to the elements of excitation-contraction (E-C) coupling becomes noticeable.

Figure 2 Postulated series of events leading to muscle damage from eccentric exercise.
During an active lengthening, longer, weaker sarcomeres are stretched onto the descending limb of their length-tension relation where they lengthen rapidly, uncontrollably, until they are beyond myofilament overlap and tension in passive structures has halted further lengthening. Repeated overextension of sarcomeres leads to their disruption. Muscle fibers with disrupted sarcomeres in series with still-functioning sarcomeres show a shift in optimum length for tension in the direction of longer muscle lengths. When the region of disruption is large enough it leads to membrane damage. This could be envisaged as a two-stage process, beginning with tearing of t-tubules. Any fall in tension at this point would be reversible. However, it would be followed by damage to the sarcoplasmic reticulum, uncontrolled Ca2+ release from its stores and triggering of a local injury contracture. That, in turn, would raise muscle passive tension. If the damage was extensive enough, parts of the fiber, or the whole fiber, would die. This fall in tension would not be recoverable. Breakdown products of dying and necrotic cells would lead to a local inflammatory response associated with tissue edema and soreness. Modified from Proske and Morgan [40].
Metabolic stress on the muscle includes increased temperature, increased acidity, insufficient mitochondrial respiration, and oxygen free radical/reactive oxygen species (ROS) production.39 ROS production has been proposed in a number of paradigms to be related to muscle inflammation and injury specifically.41 Tissue that is highly metabolically active produces ROS, which can cause irreversible damage to the cell. Disruption in calcium homeostasis can occur when the sulfhydryl groups of the ATPase pump is oxidized by free radicals, causing a reduction in the rate of calcium uptake by the sarcoplasmic reticulum. The increase in acidity that accompanies ROS production during strenuous activity affects the ability of the sarcoplasmic reticulum to take up calcium. This is due to the free hydrogen ions and the calcium ions competing for the binding sites of calcium on the ATPase pump.42
Disruption of calcium homeostasis: Disruption of calcium homeostasis is a result of the events of the initial phase of EIMD, and it occurs whether the stressor on the muscle is mechanical or metabolic. The disruption in calcium homeostasis results in a subsequent rapid activation of autogenic destructive events in the muscle fiber. It is believed that the loss of calcium from the cell plays a major role in muscle injury and subsequent repair processes.39
Calcium is necessary for normal muscle fiber function, but increased levels of calcium can be detrimental to the muscle fiber, resulting in cell dysfunction or death. The rapid increase in intracellular calcium levels is thought to be an important step in the cascade of events that result in muscle fiber damage following eccentric exercise. Increased levels of calcium in the muscle fiber results in ultrastructural changes in the muscle fiber, including inflamed and disrupted mitochondria, dilated t-tubules and sarcoplasmic reticulum, and disruption of the myofilaments.42 Data from McArdle & Jackson43 has demonstrated that an increase of calcium in the muscle fiber causes damage to the myofilaments, which is congruent with data from Byrd42 and Amelink et al.,44 whom both showed that an inhibition of calcium influx across the sarcoplasmic reticulum following exercise decreases damage in the muscle fiber. The elevated levels of calcium in the muscle fiber cause a release of enzymes through activation of phospholipase A2. This may induce injury to the sarcolemma through production of leukotrienes and prostaglandins via ROS production.39 As a result, this affects the fluidity of the membrane, and causes an efflux of intracellular enzymes and lysosomal enzymes to flow freely across the membrane.12 Increased calcium levels have also been shown to disrupt the E-C coupling process, which may be related to the decrease in force production associated with eccentric exercise.45
Autogenic processes
Following some event that initially causes some structural component of the muscle fiber to fail, meaning a physical force causes a disruption of the normal permeability barrier to calcium outside of the cell, calcium is able to enter the muscle fiber at the site of damage, and potentially overwhelm the buffering systems for calcium in the fiber. Once calcium levels in the cytosol are increased to a certain level, are elevated for a sufficient period of time, or increased within specific compartments of the muscle fiber, various degradative mechanisms are activated in the muscle fiber.1
Various processes have been postulated to explain how skeletal muscle may be damaged due to an elevation of calcium in the muscle fiber. These processes include stimulation of calcium-activated proteases, activation of lysosomal proteases, overload of mitochondria, and activation of lipolytic enzymes.12 One of the most important processes seems to be the activation of calcium-dependent proteases, specifically calpain.43 It is believed that increased levels of calcium in the muscle fiber stimulate proteases, specifically calpains, which act directly on the proteins in the membranes and specifically on the Z-discs.42,46
There are two types of calpains, type 1 and type 2, which are activated depending on the concentration of calcium in the cytosol. The type 1 isoform of calpain is activated when there are micromolar quantities of calcium, while the type 2 isoform is activated when there are millimolar amounts.47 These proteases are not specific to any protein or peptide sequence, but are associated with the degradation of particular structures in the muscle cell.48 The isoenzymes of calpain are usually localized in the I and Z bands of the muscle fiber.49 Calpain degrades Z-discs by digesting the proteins zeelin 1 and 2, which anchor α-actinin in the Z-disc.50 Calpain cleaves a variety of protein substrates including cytoskeletal and myofibular proteins, making calpain-stimulated degradation thought to contribute to the changes in structure and function of the muscle that is a result of eccentric exercise. Activation of calpain results in selective proteolysis of various contractile, structural, and metabolic elements.49
Proteins in the myofibril may also be degraded by lysosomal proteases. Increases in calcium concentration in the cytosol activate calmodulin, which is associated with lysosomal vesicles51 and phospholipase A2, which increases production of prostaglandin E2 and thereby stimulates activity of lysosomal proteases.52 Phospholipase A2 is located in the sarcolemma, membranes of organelles, cytosolic compartment, and in lysosomes.53 It is the first enzyme in the pathway that utilizes membrane phospholipids as substrate for the production of arachidonic acid, which subsequently produces prostaglandins, leukotrienes, and thromboxanes.1
Phagocytic processes
Damage to skeletal muscle initiates at what is termed the acute phase response,54 which facilitates anti-bacterial and anti-viral responses prior to promoting the clearance of debris.12 Inflammation is characterized by the movement of fluid, leukocytes, and plasma proteins in response to damage into the tissue (in this case skeletal muscle).55 Inflammatory cells migrate to the site of injury in the muscle to remove cellular debris, and myogenic cells migrate to the site of injury to initiate mechanisms to repair the damaged muscle.56 These cells penetrate the muscle by means of specific cytokines, which are small polypeptides that are considered an important link between neuroendocrinal and immunological systems that are involved in inflammation and acute phase response, among others.14,55 Specific cytokines such as interleukin (IL)-1, interferon, interleukin-2, interleukin-6, and tumour necrosis factor-α (TNF-α) are believed to be the primary mediators of inflammation.57 IL-1 is thought to influence muscle inflammation, play a role in stimulating protease synthesis and induce the expression of other cytokines such as IL-2, IL-3, IL-6, and TNF-α. TNF-α and IL-1 have been shown to have mechanisms that coincide with each other, which ultimately can lead to leucocyte adhesion and stimulating leucocyte activity.56
Leukocytes are believed to have three main functions within the damaged muscle and repair cycle: neutrophils and macrophages attack and breakdown debris; macrophages removal cellular debris; and macrophages regenerate cells.46,56 Leukocytes are drawn to injured skeletal muscle fibers through several chemotactic factors including leukocytes that are already at the site of injury, calpain fragments, and/or cytokines at the site of injury. Neutrophils are usually the first leukocytes to arrive at the site of injury. These cells then release a signal to intensify the response of other neutrophil cells and mononuclear cells.12 Neutrophils produce superoxide and ROS products via a respiratory rupture that is catalyzed by NADPH oxidase in the plasma membrane. Pyne14 determined that neutrophils do not have the ability to distinguish healthy tissue from foreign antigens, so it destroys healthy aspects of the cell as well as the damaged cell debris. While the phagocytic processes mainly include further disruption and destruction of the muscle fiber, they are necessary precursors to the regeneration phase of growth and restoration of the damaged tissue and repair of normal cell function.
Regenerative phase
During the phagocytic phase, there is a division of surviving satellite cells. These satellite cells mature into myoblasts, and fuse to form new myotubes.12 However, it is not clear how the satellite cells are stimulated to divide. Numerous studies have postulated that macrophage infiltration seems to be a critical prerequisite for regeneration, especially for proliferation of satellite cells.58‒60
Oxidative stress is a factor that is apparent in exercise-induced muscle damage. Oxidative stress is defined as the imbalance between production of ROS and the ability to detoxify reactive intermediates or to repair subsequent damage by an adequate anti-oxidant defense.61 This can lead to direct damage of cellular mechanisms by oxidation including lipids, proteins, and DNA. Furthermore, oxidative stress can act as a regulator of the acute phase inflammatory response.31 After injury, infiltrating leukocytes exert antiseptic protection of muscle by releasing ROS through oxidative burst activation of NADPH oxidase (Figure 3).62 This process results in marked perturbations of redox status in muscle fibers.63 Consequently, pro-inflammatory cytokines, including TNF-α, are released by neutrophils and injured muscle fibers, thereby activating ROS-generating enzymes such as xanthine oxidase.64 When ROS production is greater than the capacity of the antioxidant defense system, an oxidative stress occurs resulting in many cellular contents suffering oxidation due to ROS attack65 and subsequent activation of nuclear factor kappa B (NF-κB), activator protein 1 (AP-1), and forkhead box (FOXO) proteins. Additionally, increased levels of ROS results in oxidation of several proteins in the excitation-contraction mechanism,66 and the production of protein reactive carbonyl derivatives lead to a loss of catalytic activity and increased vulnerability to protein breakdown.67
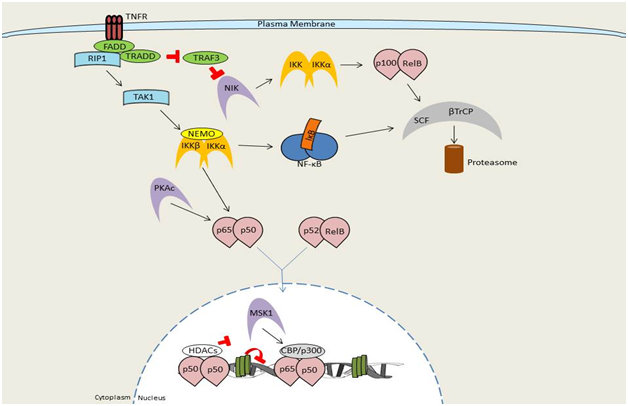
Figure 3 Overview of NF-κB signaling by TNF receptor signaling.
The classical, canonical, signaling pathway is initiated by the binding of either TNF-α, IL1, or LPS to its receptor and the subsequent sequential recruitment of the adaptors TRADD, RIP, and TRAF to the sarcolemma. IKK complex assembly and recruitment to the sarcolemma occurs between IKKα, IKKβ, and IKKγ, thereby resulting in IKKβ phosphorylation and activation. IKKβ then phosphorylates IκBα to promote polyubiquination and subsequent immediate proteasomal degradation through β-TrCP. Signaling by members of the TNF receptor superfamily, such as TNFR1, leads to recruitment of the adaptor proteins FADD and TRADD, TRAF family members (TRAF2, 5, and 6), and the kinase RIP1. The TRAF/RIP complex recruits and activates TAK1, which induces activation of the IKK complex and subsequent downstream NF-κB signaling. Upon stimulation, a subset of TNFR superfamily members that bind to TRAF3 (CD40, LTβR, BAFFR) induce TRAF3 degradation resulting in accumulation of the kinase NIK. NIK then undergoes constitutive degradation in the absence of stimulation. Accumulated NIK phosphorylates and activates IKKα. IKKα, thereby inducing processing of the NF-κB family member p100 into p52. At this point p52-containing NF-κB complexes become activated (prototypically RelB:p52).Modified from Hayden et al.62
ROS has been shown to influence the activity of transcription factor binding through various ways including activating kinases to stimulate a signaling cascade through sequential enzyme phosphorylation, regulating anti-oxidant signaling by modulating phosphatase activity, and regulating the synthesis and degradation of transcription factors.68 Allen & Tresini69 determined that almost half of the effects of ROS reported involve members of the mitogen-activated protein kinase (MAPK) and NF-κB pathways. They also concluded that hydrogen peroxide (H2O2), nitric oxide (NO), calcium (Ca2+), and cytokines are among the agents that are capable of assisting as signaling molecules in response to oxidative stress. These messengers transfer signals from the surface of the cell to the nucleus in the cell to stimulate gene expression.68 H2O2 has been indicated as the most common messenger in response to oxidative stress, contributing to more than 50% of occurrences from research studies.69
Cellular responses that are stimulated or influenced by ROS have been proposed to be classified into several categories that include modulation of cytokines, hormone release and action, growth, ion transport, transcription, and apoptosis.70 Conversely, Jackson et al.,71 proposed that cellular responses that are influenced by ROS was directly related to changes in cell proliferation, immune function, stimulation of apoptosis, or a combination of these. It can be concluded that it is still unclear whether ROS does in fact influence all, if any, of these cellular responses in skeletal muscle. Powers & Jackson72 suggest this remains unclear because the predominant area of ROS research has focused mainly on the contractile properties of skeletal muscle.
As stated previously, the NF-κB and MAPK signaling pathways are considered the most important for the cells to cope with oxidative stress. Particularly, NF-κB has been shown to be a redox-regulated factor that is most affected by ROS.73 Hansen et al.,74 determined that activation of ROS appears to involve oxidation of cysteine residues in upstream activators of NF-κB. They also showed that this is usually prevented by anti-oxidants or other reducing agents.
NF-κB is a ubiquitously expressed transcription factor that is considered vital to numerous cellular processes.75,76 It has been suggested that NF-κB directly modifies hundreds of gene products, including genes that encode cytokines, chemokines, cell adhesion molecules, growth factors, immunoregulatory receptors, acute-phase and stress response proteins, cell surface receptors, transcription factors, and several enzymes involved in protein degradation by the ubiquitin-proteasome system, as well as regulators of redox status, apoptosis, disuse atrophy, and host defense.77,78 The NF-κB family is comprised of five genes that code for protein subunits, RelA/p65, RelB, c-Rel, p50, and p52.75,76,79,80 In humans, the proto-oncogene c-Rel is a protein that is encoded by the REL gene (v-rel avian reticuloendotheliosis viral oncogene homolog). The c-Rel protein is a member of the NF-κB family of transcription factors and contains a Rel homology domain (RHD) at its N-terminus and two C-terminal trans-activation domains. The inhibitor family of NF-κB (IκB) consists of seven proteins including IκBα, IκBβ, IκBε, IκBγ, B-cell lymphoma 3-encoded protein (BCL-3), and precursor proteins p100 and p105.76,79‒81 The most commonly described forms of NF-κB are the p50/p65 heterodimer and p50/p50 homodimer complexes, followed by p50/c-Rel and p52/RelB.75,82 Unlike the other genes, p50 and p52 genes lack transcriptional activation domains, which generates p50 and p52 homodimers that function as gene repressors by blocking DNA consensus sites.75‒77,83
Prior to activation, NF-κB dimers are held in the sarcoplasm bound to inhibitory proteins, termed inhibitor of NF-κB (IκB).75,77,80 While NF-κB is bound to IκB, nuclear translocation is averted, which maintains the inactive state of NF-κB in the cytoplasm.77 However, when IκB proteins are degraded following stimulation of the cell, nuclear entry of NF-κB dimers becomes favoured.76 Specifically, once the cell is stimulated, the IκBα protein is quickly phosphorylated at serine 32 and 36, which activates poly-ubiquination and degraded by the 26S proteasome, thereby allowing nuclear entry of NF-κB dimers.80 NF-κB dimers are then free to travel to the nucleus where they bind to κB sites within target gene promoters. NF-κB, along with other transcription factors, then regulates transcription of genes.81
Activation of the NF-κB complex occurs in response to various stimuli, including infection, exposure to pro-inflammatory cytokines, mitogens, growth factors, biomechanical stressors, and oxidative stressors. There are several pathways by which NF-κB can be activated following ligation of different receptor families, including tumor-necrosis factor receptor (TNFR) and interleukin-1 receptor (IL-1R). NF-κB complex activation also includes the activation of several intermediate kinases, including NF-κB inducing kinase (NIK), which is a member of the MAPK pathways. These intermediate kinases act as distinct signaling proteins that all unite on the IκB kinase (IKK) complex.76,77
The IKK complex controls the breakdown of IκB proteins through regulation of the phosphorylation of serine 32 and 36 on IκBα, and serine 19 and 23 on IκBβ. This phosphorylation results in K48-linked polyubiquitination by the SCFβTrCPE3 ubiquitin ligase complex on lysine 21 and 22 of IκBα, which is an ATP-dependent occurrence that quickly targets the breakdown of these proteins by the 26S proteasome.75,76
It has been determined that murine C2C12 skeletal muscle cell lines contain p65/p50 in their nucleus, which is the most commonly studied form of NF-κB. In the myoblast cell line C2C12, NF-κB binds on κB sites of the cyclin D1 promoter. This then allows for transcription regulation, leading into the S phase of the cell cycle. During myogenesis, binding activity of NF-κB on cyclin D1 is decreased. This suggests that NF-κB is a critical element regulating transition to the differentiation stage from the proliferation phase. During atrophic conditions, NF-κB binds on the promoter of murine ring-finger-1 (MuRF1), which is an E3 ubiquitin ligase.84 This increases expression of MuRF1, suggesting NF-κB regulates the ubiquitin-proteasome system (UPS), resulting in muscle wasting.75,84
Another potential mechanism by which increased NF-κB activity leads to skeletal muscle wasting is the possibility of NF-κB increasing the expression of inflammation-related molecules, which either directly or indirectly enhances muscle wasting. Pro-inflammatory cytokines, including TNF-α, THF-like weak inducer of apoptosis (TWEAK), IL-1, and IL-6 are major inducers of muscle wasting. Evidence has shown that NF-κB regulates expression of cytokines, chemokines, and cell-adhesion molecules.85 It has also been determined that NF-κB inhibition promotes regeneration of skeletal muscle by limiting the inflammatory response.86
Various physiological stressors such as disease and exercise, which causes muscle injury, are known to induce oxidative stress. After injury, infiltrating leukocytes exert antiseptic protection of muscle by releasing reactive oxygen species (ROS) through oxidative burst activation of NADPH oxidase.62 This process results in marked perturbations of redox status in muscle fibers.63 Consequently, pro-inflammatory cytokines, including TNF-α, are released by neutrophils and injured muscle fibers, thereby activating ROS-generating enzymes such as xanthine oxidase.64 When ROS production is greater than the capacity of the antioxidant defense system, an oxidative stress occurs resulting in many cellular contents suffering oxidation due to ROS attack65 and subsequent activation of NF-κB, activator protein 1 (AP-1), and forkhead box (FOXO) proteins. Additionally, increased levels of ROS results in oxidation of several proteins in the excitation-contraction mechanism,66 and the production of protein reactive carbonyl derivatives lead to a loss of catalytic activity and increased vulnerability to protein breakdown.67
It is well known that cellular stress is an activator of the NF-κB classical pathway, specifically ROS formation. This has been shown in various research studies through a variety of ways that include exposure of certain cell types, such as L6 myocytes, to H2O2 leading to the activation of the NF-κB pathway; activators of NF-κB, such as TNF-α, IL-1, and LPS can all lead to an increase in H2O2 levels in the cell; and treatment with anti-oxidants, such as glutathione (GSH), prevents NF-κB activation.68 Sen et al.,87 were the first to demonstrate that TNF-α-induced activation of NF-κB in L6 myocytes, which was augmented by conditions of oxidative stress. It was also determined that inhibition of NF-κB activity by the antioxidant pyrrolydinedithiocarbamate, coupled with an increase in intercellular adhesion molecule -1 (ICAM-1) expression, suggested that the intracellular redox status was critical in activation of NF-κB, nuclear translocation, and subsequent gene transcription regulation of proteolytic genes.88 Li et al.,79 also found that TNF-α led to ROS-mediated NF-κB activation, resulting in a decrease of total protein in skeletal muscle with a specific loss of myosin heavy chain. The enhanced muscle protein degradation was coupled with TNF-α-dependent stimulation of total ubiquitin conjugation of myotube proteins.79
The NF-κB signaling pathway has been the most widely studied pathways involving regulation of the redox-sensitive pathways.89 However, the NF-κB signaling pathway works in conjunction with the MAPK signaling pathway and its proteins ERK, JNK, and p38 specifically, which will be briefly noted.
The main function of the MAPK pathway is to facilitate growth, metabolism, transcription, differentiation, translation, and remodeling. MAPK is known to have a complicated hierarchy that is intertwined with other kinases, such as ERK and JNK, which are up-regulated by their own respective kinases.69 The main activators of the MAPK pathway include growth factors, inflammatory cytokines, and phorbol esters. In the ERK and JNK pathways, Ras has a critical role in the activation of MAPK. Ras stimulates the translocation of Raf-1, which controls MAPK kinase (MEK/MKK).68 TNF-α and IL-1 are able to bypass the Ras pathway through increasing the level of H2O2 in the cytosol, which activates several forms of protein kinase C (PKC).90 It has been suggested that PKC is a pivotal enzyme activating MAPK pathways through stimulation of MEK/MKKs, as well as the NF-κB pathway through NIK activation.68 This shows the importance of crosstalk between the NF-κB pathway and MAPK pathways, since both pathways may directly or indirectly affect each other.
MAPK can be activated by cytokines and stress, which stimulates inflammation, degradation by phospholipase A2, and apoptosis.69 In this regard, MAPK regulates gene expression mainly under the control of NF-κB signaling, such as through anti-oxidant enzymes. It is also worth noting that adaptations in skeletal muscle such as mitogenesis, hypertrophy, and transformation of fibers are regulated by MAPK signaling, and have been shown to play a critical role in determining homeostasis of cellular oxidant-anti-oxidant mechanisms.68
Recently, the potential protective role of estrogen from muscle damage has been of growing interest. However, it is still unclear as to whether estrogen really does play a protective role of muscle damage in humans. Numerous animal studies have supported the suggestion that estrogen has the potential to alleviate indicators of EIMD and inflammation. Conversely, human research has yielded more conflicting results. The discrepancies in human research of estrogen and muscle damage has mainly been attributed to differences in age, fitness levels, exercise protocols, and focus on sex-based differences as opposed to estrogen-specific effects.25 Further research is needed in this growing area of interest.
Estrogens describe a group of 18-carbon steroids molecules that are secreted mainly by the ovaries in females, and the testes in males to a much lesser extent.91 While estrogens are mainly involved in the development and maintenance of normal reproductive and sexual function, they also have other biological effects in various physiological systems such as the cardiovascular system, musculoskeletal system, immune system, and central nervous system.92 Estrogen refers to three steroid hormones that are similar in structure that include estradiol-17β (E2), estrone (E1), and estriol (E3).12 Of these three steroid hormones, E2 is the main estrogen in humans, as well as the one with the most estrogen-like properties.93 The other two estrogens, E1 and E3, have been shown to be more tissue-specific and are found in much smaller quantities than E2, making them less studied in humans.94
The protective role of estrogen has already been demonstrated in various physiological systems in humans. With respect to skeletal muscle, estrogen has been shown to exert protective effects, but the mechanisms by which this occurs remains unclear. Three hypotheses have been proposed to explain estrogen’s influence on skeletal muscle: 1) estrogen is thought to have a high anti-oxidant capacity, and may have the ability to forage ROS and stimulate the expression of anti-oxidant enzymes, which limits oxidative damage;95 2) estrogen has a similar structural property to cholesterol, possibly allowing it the ability to intercalate within membrane phospholipids similar to cholesterol and exert a membrane-stabilizing effect;93 and, 3) the discovery of three types of estrogen receptors (ERα, ERβ, and plasma membrane ER) has led to the determination that estrogen may have the ability to have gene regulatory effects.96 However, less seems to be known about the latter two hypotheses.
Estrogen is believed to have anti-oxidant characteristics due to the fact that its molecular structure is based on a carbon-ring structure, originating from a phenol species. Phenol species have at least one, or more, hydroxyl groups that give them the ability to reduce electrons.97 Lipid peroxidation, which is a chain reaction that is mediated by ROS, can be stimulated by the hydroxyl radical attacking polyunsaturated fatty acids in membranes, resulting in oxidative damage93 that affects the stability of the membrane. Numerous studies have shown that estrogen has anti-oxidant properties;93,98‒104 however, the mechanisms by which this occurs is not completely understood. It is believed that since estrogens possess a hydroxyl group on their phenolic ring in the same configuration and position as vitamin E, they may donate hydrogen atoms from the phenolic hydroxyl group, which would cease peroxidation chain reactions in a similar fashion to vitamin E.105,106
However, there is conflicting evidence as to whether estrogen has potential anti-oxidative protective effects. A study by Paroo et al.,107 demonstrated anti-oxidative properties of estrogen following running exercise, while Feng et al.,108 and Stupka & Tiidus109 both demonstrated anti-oxidative properties of estrogen following muscle injury. Conversely, Tiidus et al.,110 did not demonstrate anti-oxidative effects of estrogen in post-exercise indicators of oxidative stress. They also showed that estrogen might reduce levels of other anti-oxidants, such as vitamin C and glutathione, in some muscle and tissues, arguing the point that estrogen may not exhibit anti-oxidative properties. Enns & Tiidus25 suggest the inconsistencies of research in this area, particularly in humans, is likely due to the examination of chemical indicators of post-exercise oxidative stress in the blood as opposed to muscle biopsies, and focus on sex-based differences rather than the effects of estrogen. However, there is more evidence to support the argument that estrogen does exert anti-oxidative effects. Dernbach et al.,111 showed that female rowers had lower levels of an oxidative stress marker in the blood after a 4-week training program compared to their male counterparts, while Ayres et al.,112 demonstrated that amenorrhoeic females showed a significantly greater potential for lipid peroxidation following an acute bout of exercise compared to eumenorrhoeic females. Data has also been presented that suggest females may be protected more at certain points in their menstrual cycle. Kerksick et al.,32 determined that females at the mid-luteal phase of their menstrual cycle had higher serum concentrations of superoxide dismutase (SOD), an anti-oxidant enzyme, compared to males following eccentric exercise. However, further research is needed in this area, as there are still questions and conflicting data.
Due to estrogen’s potential anti-oxidant ability and configuration, it is thought to have membrane-stabilizing characteristics.12 Wiseman & Quinn113 have suggested that estrogen may decrease membrane fluidity and increase membrane stability to protect them from peroxidative damage in a similar fashion to cholesterol, which may be a mechanism of estrogen’s anti-oxidant activity. Since steroid hormones are lipophilic, they can intercalate into the bilayer of the cell plasma membrane, which would potentially alter the fluidity and function of the membrane.12
As previously stated, pro-inflammatory cytokines increase following exercise, during muscle damage and the repair cycle. Yoshikawa & Yoshida114 determined that vitamin E could inhibit NF-κB, which governs gene expression of various cytokines. They demonstrated that vitamin E prevents leukocyte-endothelial cell adhesion of inhibiting signaling transduction. Thus, they concluded vitamin E could have a protective effect against inflammation progression. Since estrogen has a similar structure and configuration to vitamin E, Kendall &Eston12 suggest that estrogen could affect the expression of adhesion molecules, and potentially assuage any further damage by reducing neutrophil infiltration. However, in doing so, they also state this would inhibit the necessary inflammatory processes that leads to regeneration of the cell.
Creatine kinase is found in the cytosol and mitochondria of tissues where energy demands are high. There are two types of subunits of CK: muscle type (M) and brain type (B). These two subunits form three tissue-specific isoenzymes: cardiac muscle (CK-MB), skeletal muscle (CK-MM), and brain (CK-BB). CK forms the core of the phosphocreatine (PCr) system. In the PCr system, the cytosol isoenzymes are closely linked to glycolysis and produces ATP.115 The mitochondrial CK (MtCK) is closely linked to the electron transport chain (ETC) and can use ATP in the mitochondria to regenerate PCr. This system is important for the production and maintenance of energy supply, and is involved in the metabolic feedback regulation of respiration.116 Skeletal muscle CK can account for as much as 20% of the soluble sarcoplasmic protein in muscle.115
One of the most common markers of muscle damage, specifically muscle membrane disruption, is the appearance of CK in the blood.117 A significant difference in CK activity has been shown between males and females at baseline and following muscle injury. In these studies, this difference has been attributed to estrogen effects. Amelink & Bär118 showed that CK levels were significantly higher in male rats compared to female rats following muscle injury, which they attributed to the presence of estrogen. Further data from this group also showed a direct inverse relationship between estrogen supplementation and CK release in normal male and female rats, as well as ovariectomized female rats.119 Data from this group suggest that the membrane-stabilizing effects of estrogen may assuage post-exercise CK release from skeletal muscle. They also showed that circulating CK levels could indirectly reflect changes in exercise-induced muscle membrane disruption. However, it is unknown whether the reduction in efflux of CK is an indication of increased membrane stability or if, in fact, the muscle is receiving less damage.
It has been suggested that since estrogen may be responsible for differences in gender-related vulnerability of muscle to EIMD, and may act as an anti-oxidant, the effects of estrogen administration on phagocytic infiltration into the muscle fiber following exercise should be examined.120 Tiidus & Bambardier120 hypothesized that a decrease in infiltration of neutrophil and macrophages may reduce the time-course and severity of the inflammatory response of the muscle following exercise, and potentially boost regeneration. They measured post-exercise tissue myeloperoxidase activity in male and female rats that were supplemented and not supplemented with estrogen. Their results showed that female rats had significantly attenuated infiltration of neutrophils into skeletal muscle 24-hours post-exercise when compared to male rats. However, when the male rats were supplemented with estrogen, they showed the same attenuation of neutrophil infiltration 24hours post-exercise as the female rats. These results suggest that estrogen significantly affects post-exercise infiltration of leukocytes into skeletal muscle. Numerous other data from these researchers have confirmed these results.96,109,121,122 However, the mechanism(s) via which this occurs is still unclear.
St. Pierre Schneider et al.,123 examined the time course and leukocyte concentration in injured soleus muscle in male and female mice to determine if gender differences were present. They determined that leukocyte invasion began one day following injury, and was greatly reduced on the fifth day post-injury in males, but persisted until the seventh day in female mice. Maximal leukocyte infiltration was seen to occur on the first day post-injury, and muscle sections obtained from male mice contained more fibers infiltrated by acid phosphatase-positive cells than muscle sections obtained from female mice. The difference seen between genders was suggested to be due to estrogen preventing an increase in macrophage concentrations in blood vessels by limiting the availability of endothelial cell adhesion molecules. They suggested that estrogen could decrease leukocyte migration into inflamed tissue because fewer endothelial cell adhesion molecules result in the failure of leukocytes to move out of the blood vessels and into the inflamed tissue. These results suggest that removal of damaged myofibers is slower in females than in males.
Tiidus124 and Tiidus et al.,121 have demonstrated that estrogen may protect skeletal muscle from muscle damage and inflammation, specifically neutrophil infiltration, following exercise through inhibition and stabilization of calcium-activated calpains. Since it is believed that estrogen can act as a membrane stabilizer, it may act to minimize the disruption of the membrane during injury, which prevents the influx of calcium down its concentration gradient.25 This would result in a decrease in calpain activity, and would prevent any further damage from occurring. Also, since muscle proteolysis by calpains attracts pro-inflammatory cells such as neutrophils, estrogen is suggested to protect muscle from further damage by inhibiting the recruitment of inflammatory leukocytes into muscle.120 Tiidus et al.,121 confirmed this hypothesis in an investigation that demonstrated a significant attenuation of 1-hour post exercise nuetrophil concentrations and myeloperoxidase activity in ovariectomized rats given estrogen supplementation. They also showed a reduction in calpain-like activity compared with ovariectomized rats treated with placebo. This study suggested that supplementation of estrogen, increased stability of the sarcolemma post-exercise, which prevented the activation of calpain.
Estrogen has also been suggested to influence pro-inflammatory cytokines; although, the relationship is complicated. Angstwurm et al.,125 determined that during the follicular phase of the menstrual cycle, an increase in E2 was complemented with an increase in IL-6. Also, when progesterone levels rose following ovulation, a 1.5- to 4.4-fold decrease in plasma IL-6 was seen. Schwarz et al.,126 demonstrated that male participants showed no difference in cytokine response between baseline samples and samples taken one to three weeks later. They also showed that TNF-α and IL-6 was significantly decreased in the luteal phase in pre-menopausal females, compared with their male counterparts, with the difference being more pronounced in females taking oral contraceptives. A diminished response during the luteal phase compared with the follicular phase was also seen. These results suggest that estrogen exhibits an inhibitory effect on activation of pro-inflammatory cytokines. However, Schwarz et al.,126 also demonstrated a positive correlation between estradiol concentrations in plasma and the release of TNF-α and IL-6 following a challenge during the luteal phase.
Little is known about the potential of estrogen to stimulate processes of regeneration of muscle such as satellite cell activation and proliferation. A study performed by McClung et al.,127 reported re-growth and regeneration of skeletal muscle following a period of atrophy in rats is dependent on estrogen status. Also, differences in gender have been observed in satellite cell activation and proliferation. For example, Roth et al.,128 demonstrated that, after nine weeks of resistance training, women showed a greater increase in the number of satellite cells in the vastuslateralis muscle than men. Tiidus et al.,122 reported male rats that were supplemented with estrogen had an increase in satellite cells 72hours following a session of downhill running. In a follow-up study, they attempted to determine which stage(s) of the satellite cell cycle was influenced by estrogen. Following a similar protocol to the prior study, ovari ectomized female rats were supplemented with either estrogen or a placebo, and the researchers examined the histochemical changes in numbers of total satellite cells (Pax-7 positive), activated satellite cells (MyoD-positive), and proliferating satellite cells (BrdU-incorporated).129 The results showed significant increases in all three of the observed markers. Therefore, they concluded that sex-mediated differences in muscle fiber regeneration and satellite cell numbers might be directly related to the influence of estrogen, and estrogen might have an influence on satellite cell activation in muscle following a bout of exercise through upstream mechanisms.
While the mechanisms by which estrogen influences satellite cell activation and proliferation are unknown, Enns & Tiidus25 have suggested various receptor- and non-receptor-mediated roles for estrogen that may exist. Enns et al. showed that estrogen receptors play a critical role in influencing muscle repair processes through increases of satellite cell activation and proliferation. Blocking estrogen receptors was shown to completely eliminate both exercise- and estrogen-mediated increases in all three of the satellite cell markers.96 Other data was able to pinpoint which estrogen receptor (ER-α) is specifically responsible for estrogens influence on satellite cells.130
It has also been suggested that, in the presence of estrogen, various downstream signaling pathways and targets of estrogen receptor binding exists that might be responsible for post-exercise up-regulation of satellite cells.25 For example, Patten et al.,131 and Sitnick et al.132 demonstrated that through binding of estrogen to estrogen receptors, the PI3K/Akt pathway stimulates growth and protein synthesis. In addition, Kahlert et al.,133 demonstrated that 17β-estradiol promotes cell growth via the estrogen receptor-mediated induction of the early genes c-fos and egr-1 in myoblasts.
While the potential cyto-protective role of estrogen as an anti-oxidant has been of growing interest in the field, the use of fish oil as an anti-oxidant has been well established. Fish oil is part of the polyunsaturated fatty acids (PUFAs) family, and can be classified as omega-3 fatty acids (n-3) or omega-6 fatty acids (n-6) based on the location of the last double bond relative to the terminal methyl end of the molecule.134 The precursors of n-3 and n-6 series of fatty acids are known as α-linolenic acid (ALA) and linoleic acid (LA), respectively. These are essential nutrients of the diet; however, the body is unable to produce these fatty acids.
Numerous studies have shown that n-3 attenuates the inflammatory response during muscle damage through the production of eicosanoids, which are mediators of inflammation, mainly through EPA and DHA. Dietary fish oil has been shown to result in a decrease in leukocyte chemotaxis, decreased production of ROS and other pro-inflammatory cytokines, and decreased adhesion molecule expression. The majority of studies involving fish oil supplementation have been focused on the protective effects of fish oil on the cardiovascular system; however, the possible effects of fish oil attenuating exercise-induced muscle damage is of growing interest. While the majority of studies on this subject have shown positive results, there are still some inconsistencies in the research, furthering the need for additional research in this area.
Fish oil derived n-3 fatty acids have been shown to have numerous benefits on health status that vary from cardiovascular benefits to slowing the progression of specific cancers.135 PUFAs have also been shown to play a role in the regulation of the inflammatory response through the production of eicosanoids, which are inflammatory mediators.136 Eicosanoids mediate the inflammatory response through production of pro-inflammatory and/or anti-inflammatory eicosanoids production. The production of pro-inflammatory eicosanoids prostaglandin E2 (PGE2), and leukotriene B4 (LTB4) are derived from the n-6 fatty acid arachidonic acid,137 while the anti-inflammatory eicosanoids EPA and DHA are derived from n-3 PUFAs.134
Biochemically, n-3 fatty acids are a class of PUFAs that have the first carbon-carbon double bond in the third position from the methyl end of the fatty acid, which is the n-3 position, with additional double bonds depending on the molecule. EPA is a longer chain n-3 fatty acid with a 20-carbon chain and 5 double bonds, and DHA with a 22-carbon chain with 6 double bonds. Both EPA and DHA are found in cold water fish that include salmon, mackerel, sardines, and herring. ALA is the shortest chain form of n-3 and only contains 18-carbon atoms with 3 double bonds, and can be found in plant sources such as flaxseeds, soybeans, and walnuts.138 ALA can be converted into longer chain derivatives, and is therefore known as a precursor to other n-3 fatty acid chains. However, conversion from ALA to EPA and DHA is very inefficient and difficult to estimate an exact percentage,139 with percentages ranging from 5-20% being converted to EPA and about 0.5-9% converted to DHA.139,140
Metabolism of ALA to EPA begins with the conversion of ALA to stearidonic acid, which is then elongated to eicosatetraenoic acid. The eicosatetraenoic acid formed is then converted to EPA, which is then either metabolized to DHA or to eicosanoids via enzymes cyclooxygenase (COX) and lipoxygenase (LOX). Conversion of EPA to DHA occurs with the addition of two carbons to form docosapentaenoic acid, the addition of two additional carbons to form tetracosapentaenoic acid, desaturation to form tetracosahexaenoic acid, and finally removal of two carbons by limited β-oxidation to produce DHA.
The pathway of elongation of n-6 and n-3 fatty acids occurs in the liver. Both LA and ALA are metabolized by the same enzymes, resulting in a competition between the LA and ALA; the one with an excess causes a decrease in metabolism of the other fatty acid.134 Although, when the ratio of n-6 fatty acids and n-3 fatty acids is 1:1, the desaturases and elongases seem to exhibit an affinity to metabolize n-3 over n-6 fatty acids.141,142 Usually, however, the ratio is in favor of n-6 fatty acids, which results in a greater conversion of LA to arachidonic acid.143 Several studies have shown that increasing ALA intake to at least 4.5grams/day appears to produce a substantial increase in EPA plasma phospholipid content.144‒146 Decreasing LA in the diet has also been shown to increase metabolism of ALA to its longer chain derivatives.147
Eicosanoids include prostaglandins (PG), thromboxanes (TX), leukotrienes (LT), and hydroxyeicosatetraenoic acids (HETE). These eicosanoids are derived primarily from 20-carbon PUFAs arachidonic acid and EPA, which are important inflammation regulators.148 Specifically, these regulators control the intensity and duration of the inflammatory responses.149 Although, the resulting physiological response depends on the types of cells present, the stimulus, the timing and concentration of eicosanoid production, and the sensitivity of the target cells.150 As stated previously, there is a competition between n-6 and n-3 fatty acids since they utilize the same enzymes. This competition occurs at the enzymes COX and LOX.134 Eicosanoids derived from n-6 are pro-inflammatory including PGE2, which induces production of IL-6 in macrophages and causes pain and vasodilation, and LTB4, which leads to the production of inflammatory cytokines such as TNF-α and IL-1 by macrophages.148 Conversely, eicosanoids derived from n-3 are anti-inflammatory,151 and act as a substrate for COX and LOX. This results in the production of the 3-series PGs and TXs, the 5-series LTs, and the hydroxyl-EPAs.134
Increased consumption of EPA and DHA has been shown in numerous studies to result in an increased concentration of those fatty acids in inflammatory cell phospholipids. An increased consumption of these fatty acids also has been shown in numerous studies to result in a decrease in the pro-inflammatory eicosanoids and an increase in anti-inflammatory eicosanoids. This suggests that the mediators that are formed from EPA and DHA are less potent than those mediators that are formed from arachidonic acid.136 Several studies have identified a group of mediators that are formed from EPA and DHA by COX-2, termed E-series resolvins and D-series resolvins, respectively, also appear to have anti-inflammatory effects.152,153
There have been a few studies showing that n-3 fatty acid supplementation decreases ROS production, specifically H2O2 and superoxide, through stimulation of neutrophils. For example, Luostarinen & Saldeen154 showed a significant decrease in superoxide generation by neutrophils without the involvement of COX or without altering neutrophil lysosomal enzyme release in 12 men that were supplemented with 5.4 grams of EPA and 3.2 grams of DHA daily for four weeks. Fisher et al.,155 also showed that supplementation with 6 grams of EPA and DHA for 6 weeks decreased H2O2 production in monocytes. However, numerous studies that have utilized lower doses of EPA and DHA of less than 2.3 grams per day failed to demonstrate any effect on ROS production by neutrophils or monocytes.156‒158 Therefore, it is suggested that n-3 fatty acid supplementation may only be effective to attenuate ROS production at higher doses.
Long chain n-3 fatty acids exert another anti-inflammatory effect that is mediated at altered inflammatory gene expression through effects on transcription factors such as NF-Κb.159 As stated previously, NF-κB is a transcription factor that plays a critical role in inflammatory signaling pathways. Several studies have shown that EPA inhibits the activity of NF-κB through a decrease in the degradation of IκB, which is an inhibitory subunit of NF-κB.160‒162
Numerous studies have hypothesized potential mechanisms by which n-3 supplementation may increase the benefits of exercise. One potential mechanism is by increasing lipolysis and β-oxidation. It has been suggested that n-3 fatty acids act as metabolic fuel partitioners163 by up-regulating lipid oxidative enzymes and down-regulating lipogenic gene expression.164,165 Several studies have claimed that this is in part due to the ability of n-3 fatty acids to bind and activate various peroxisome proliferator-activated receptor (PPAR) isoforms, which are members of the nuclear receptor superfamily.166,167 Diep et al.,168 determined that n-3 fatty acids have a higher affinity to act as ligands for the PPAR-α isoform, which is found in the nucleus of cells of many body tissues that exhibit high oxidative rates of fatty acids, including skeletal muscle. Theoretically, an increase in the activity of PPAR-α should facilitate an increase in the reliance on fat as a fuel source during exercise, thus sparing muscle glycogen and improving performance.138
Another mechanism in which n-3 fatty acid supplementation may increase the benefits of exercise is by increasing fatty acid delivery to the working muscles through an increase in blood flow.169 It has been suggested that this improvement in blood flow is due to n-6 eicosanoid production being suppressed by n-3 fatty acids, leading to a decrease in pro-inflammatory eicosanoids, which cause vasoconstriction and an increase in anti-inflammatory eicosanoids, which allows for vasodilation.138 It has also been suggested that n-3 fatty acids supplementation may prevent red blood cells from deformity, which may be attributed to an increase in the peroxidation of lipid membranes that occurs as a result of ROS production.170
Fish oil supplementation has been widely studied during aerobic exercise as well as in various disease states; comparatively, there are very few studies that focus on fish oil supplementation and the effects on eccentric exercise and inflammation. As stated previously, muscle weakness and soreness are common occurrences of EIMD. Research has shown that following a quick recovery within 2 to 3 hours following exercise, muscle strength slowly returns to baseline, but may remain depressed for a few days or weeks, depending on the degree of damage.171,172 During this time, structural damage and subsequent inflammatory events may progress before muscle repair and regeneration occurs. EIMD is usually accompanied by DOMS, but the mechanisms are not fully understood. Phillips et al.,173 determined that DHA supplementation reduced the exercise-induced inflammatory response that occurs following eccentric exercise. Tartibian et al.,174 hypothesized that the supplementation of n-3 fatty acids would result in an anti-inflammatory response to exercise, which may subsequently reduce DOMS. They proved their hypothesis correct by demonstrating that 1.8 grams of n-3 fatty acid supplementation in healthy males reduced pro-inflammatory eicosanoids such as IL-6, PGE2, and TNF-α following eccentric exercise. In a previous study, Tartibian et al.,34 showed that ingestion of 1.8 grams of n-3 fatty acids decreased DOMS in men following eccentric exercise. More recently, Lembke et al.,175 showed that supplementation of 2.7grams of n-3 fatty acids for 30 days could reduce DOMS and C-reactive protein (CRP) following eccentric exercise when compared to sunflower oil. Jouris et al.,35 determined that following ingestion of 3 grams of n-3 fatty acids for 7days, DOMS was significantly reduced in 3 males and 8 females following eccentric exercise. Considering the results of these studies, Kim & Lee176 suggested that an amount of 1.8 to 3 grams should be sufficient to effectively reduce DOMS following eccentric exercise.
Several studies have also measured oxidative stress markers to explain the potential reason n-3 fatty acids reduce DOMS, but the results have been inconsistent among the studies. For example, one study determined that 1.8grams of n-3 fatty acid supplementation for 30days did not significantly reduce DOMS and malondialdehyde (MDA) following exercise,26 while another study reported that 3 grams of n-3 fatty acid ingestion for 6 weeks significantly reduced thiobarbituric acid-reactive substance (TBARS), which is a marker of lipid peroxidation, when compared to a placebo.36 However, Gray et al.,36 did not determine that there was a difference in DOMS between the experimental group and the placebo group. These results suggest that n-3 supplementation is more associated with the inflammatory response as opposed to with oxidative stress for decreasing DOMS following eccentric exercise.
While the exact mechanisms of EIMD, and how estrogen and fish oil may potentially play a protective role in the process of muscle damage has yet to be clearly elucidated, this area of research is becoming of greater interest to researchers. More research needs to be conducted to objectively determine the exact mechanisms of EIMD, and how muscle damage may potentially be attenuated either with estrogen or other anti-oxidants such as fish oil. The hypotheses that have been suggested thus far have shown promise, with the majority of researcher data supporting their claims; however, more research must been done to be able to confidently confirm them. Estrogen and fish oil are thought to attenuate EIMD mainly during the inflammatory phase of muscle damage. However, the inflammatory process may be necessary to allow muscle to adapt, regenerate, and repair. Further research must be conducted to determine if this is indeed the case. While this area is of growing interest, very little is concretely known about these processes and mechanisms, and further research is obviously warranted to determine the exact mechanisms that are involved.
None.
Author declares that there is no conflict of interest.

©2015 McKinley-Barnard, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.