Journal of
eISSN: 2373-4310


Review Article Volume 5 Issue 4
Department of Life Sciences, University of Baghdad, Iraq
Correspondence: Mays Imad Ahmed, Department of Life Sciences, Faculty of Science, University of Baghdad, Iraq
Received: January 27, 2016 | Published: December 15, 2016
Citation: Mahmod MEA. Immobilization of Bacillus Subtilis glutaminase on different support is. J Nutr Health Food Eng. 2016;5(4):668-670. DOI: 10.15406/jnhfe.2016.05.00179
Five Bacillus sp were obtained from soil, one isolate was selected according its, highest enzyme productivity, it was identified as Bacillus BG. The bacteria was cultured in a liquid medium, the enzyme precipitated by 30% saturation of ammonium sulphate, dialyzed and immobilized by adsorption on different supports including sephadexG-25, DEAE-cellulose, chitin powder, sawdust, charcoal.
DEAE-cellulose retained most of enzyme activity (90%) followed by chitin (78%), then sephadex G-25 (73%) while sawdust and charcoal retained (55%) only. The immobilized enzyme was subjected to different temperatures charcoal was the best matrix for protecting glutaminase against heat, it retained 55 and 22% of original activity chitin after 2hrs of incubation at 50 and 60°C respectively while the free enzyme retained 30 and 10% at the same condition.
The immobilized enzyme was more stable at Ph 8 than at pH 5. The enzyme adsorbed on DEAE-cellulose retained the maximum activity (98%) at pH 8 for 2hrs, while it was 73% for the free enzyme. It can be concluded from these results thatBacillus subtilis glutaminase can be immobilized on different inert materials, Sephadex G-25 is more suitable in this project. In general the immobilized enzyme is more stable at different temperatures, pH and time than the free enzyme.
Keyword: Pseudomonas aeruginosa, glutaminase, enzyme, sawdust, charcoal, small intestine, cancer treatment, immobilization
Glutaminase (EC.3.5.1.2), it is found in many in many eukaryotic and prokaryotic cell, glutaminase can be used in food industries as flavor enhancer, pseudomonas fluorescens and vibiro casticola may be added to some kinds of food to give a popular flavour.1 Glutaminase is used in oral dehydration solution since it transforms glutamine which helps in absorption of ions in small intestine.2 It has many advantageous in food and pharmaceutical application, L-glutaminase is used in cancer treatment.3
The attachment of enzymes to inert materials is a kind of immobilized method, it is practical and suitable method for many industrial application4 there are many advantageous for the immobilization of cell and enzyme, such are isolation and purification of enzyme is obviated, reduces the expense of the free enzyme since the immobilized enzyme can be used for many reaction cycle, increasing the stability of enzyme toward the environment conditions such as PH, temperature and ionic strength, reduces the volume of bioreactors and other benefits.5
This study is to determine to immobilization Bacillus subtilis glutaminase and the stability of immobilization at different condition, which may give many practical and economic advantage.6
Collection of samples
Five sample were collected from soil and water, 0.1 of each samples was spread on the surface of nutrient agar plates and inoculated at 37˚C for 24hr, the growing colonies were purified by sub culturing on nutrient agar for many times until pure culture was obtained.
The morphology, size, shape and margin of the bacteria isolated on the nutrient agar plate were studied. On the other hand a loop-full of bacterial suspension was fixed on slide and studied by Gram stain to examine Gram reaction and shape.7
Growing of bacteria in glutamines production medium
Bacterial isolates were activated in N.B for 18hrs 2ml of this culture was added to 100ml of glutaminase liquid medium8 and incubated at 37°C for 24hrs in shaking incubation at 100rpm.
Assay of glutaminase activity
Bacterial cells were precipitation by centrifugation at 6000rpm for 15min the precipitate was collected and washed twice with 0.02M phosphate buffer PH 8. Fifty micro liters of cell suspension was added to 200micro liters of 0.1M 30min in shaking water bath the reaction was stopped and glutaminase activity.9 One unit of enzyme activity is the amount of enzyme required for production of one micromol of ammonia from glutaminase under reaction condition.
Extraction of glutaminase from bacteria cell
Bacteria cell were harvested from the production medium and washed as described above, and then they were disintegrated by ultrasonication for 5min under cold condition.10 The solution was centrifugation at 8000rpm for 30min glutaminase was measures in supernatant.
Precipitation of glutaminase by ammonium sulphate
Glutaminase was precipitation by 30% saturation of ammonium sulphate under cold condition and dialyzed against D.W for 24hrs.
Immobilization of the enzyme
Different solid were used for immobilization of glutaminase such: charcoal, DEAE-cellulose, sawdust, sephadexG-25 and chitin, these material were autoclaved at 121°C for 30min and dried at 90°C in the oven for 24hrs.
Solution of glutaminase was added to these material 1:1 v:w, and incubation for 48hrs, the suspension were filtration whatman no 1 paper, the material on the filter paper washed with 0.01phosphat buffer to remove unbounded enzyme.
Assay of immobilization enzyme activity
0.2gm of the immobilization enzyme was placed in beaker, 5ml solution glutaminase added, incubated at 37°C for 1hr in shaking bath and enzyme activity was assayed after different of suspension.
Determination of temperature effect on immobilization enzyme
Immobilized enzyme was incubated at 37,50,60 for 30,60,90 and 120 min after which transferred to ice bath filtered and activity of glutaminase was determined. The free enzyme was treated in the same procedure.
Five bacterial were obtained and identified as according to morphological and growth color on the nutrient agar medium. The result showed that the activity of in liquid medium ranged between 1.5-3.9U/m.
One isolate was selected according to its higher enzyme activity than others, it was identified as Bacillus subtilis BG5 (Table 1). Many kinds of bacteria and fungi can produce glutaminase which be intracellular or extracellular glutaminase important role in cell metabolism.10,11
Code Number of Isolates |
Zone of Lysis mm |
BG1 |
0.5 |
BG2 |
1.3 |
BG3 |
1.6 |
BG4 |
2.4 |
BG5 |
3.1 |
Table 1 Bacillus subtilis isolates and there in medium incubation at 37˚C for 48hrs
Immobilized glutaminase
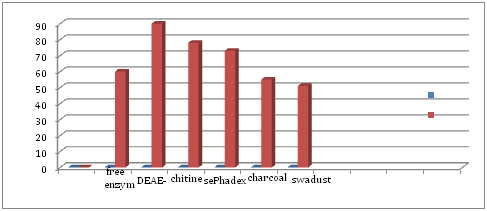
Bacillus subtilis BG5 glutaminase was adsorbed on different solid material DEAE-cellulose the maximum enzyme activity was 90% followed by chitin 78%, then sephadex G-25 73% while sawdust & charcoal 51% (Figure 1). The high remaining activity for DEAE-cellulose immobilized to porous of material good surface for adsorption of enzyme particles. Glutaminase adsorption chitin is the best surface to adsorption.12
Effect of temperature on immobilized glutaminase
Bacillus subtilis glutaminase immobilized with DEAE-cellulose, chitin powder, charcoal selected to study the influence of temperature and PH. Different chemical compound DEAE-cellulose an organic polymers, chitin inter-material and charcoal carbonaceous material DEAE-cellulose immobilized with glutaminase retained 87% of the original activity when incubation at 37°C for 120min. While 72% for free enzyme and 70% for each chitin and charcoal. The activity of glutaminase decreased at 55°C and 65°C it was with (3kinds of supports). All more stable than free enzyme (Figure 2A-2C).
Chitin is best for protect glutaminase against heat retained 50 and 18% activity for 24hrs at 55°C and 65°C followed charcoal retained 40 and 10% respectively while the remaining activity DEAE-cellulose 42% at 55°C and 15% at 65°C compared with 30 and 10% for free enzyme at conditions.
It can be form these result glutaminase of Bacillus subtilis BG5 is heat not stable but when immobilized become more resistant, this point many advantages industrial application when use high temperatures. Glutaminase of Zygosaccharomyces rouxii immobilized in hydro gel matrix stay activity 50-70°C,13 while Aspergillus awamori immobilized with sepharose matrix stag activity 50-60°C.14
Was obtained five isolates of bacteria Bacillus subtilis and studied the ability of isolates to produce Alklotames, and results showed qualitative and quantitative screening that isolation Bacillus subtilis BG5.
Failed enzyme using ammonium sulphate by 30% and has Delzath, then under adsorption on different materials included Dolly DEAE-cellulose), Alcaatin powder and sawdust, coal sephadex G-25).
The results showed that DEAE-cellulose, retains most of the effectiveness of the enzyme Alcaatin followed by 90% to 78% and then sephadex G-25 to 73% while retaining sawdust and coal for only 55% of the effectiveness of the enzyme. Were exposed enzyme free and unrestricted different degrees of heat, It was observed that the enzyme restriction is more stable than the free enzyme at different temperatures, showed Alcaatin powder good protection for the enzyme at high temperature as remains retains 55% and 22% of the original efficiency when exposed to 50°C and 60°C for two hours respectively where kept enzyme free 30 and 10% of these Class. Louhz that enzyme unrestricted firmer number pH 7 than No. 5 and keep the enzyme unrestricted b DEAE-cellulose highest ratio of 98% at pH 8 for two hours while retaining the enzyme free to 73% in the same conditions.
We conclude that the enzyme Alklotamnez product of bacteria Bacillus subtilis can be restricted by way of adsorption on different materials, and DEAE-cellulose material suitable for Qidah at the top of effectiveness while Alcaatin protects the enzyme from high temperature and different pH values.
None.
Author declares that there is no conflict of interest.

©2016 Mahmod. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.