Journal of
eISSN: 2373-4310


Research Article Volume 11 Issue 1
1Biology Department, Frostburg State University, USA
2Health Science Department, Frostburg State University, USA
3Chemistry Department, Frostburg State University, USA
Correspondence: Kumudini Apsara Munasinghe, Biology Department, Frostburg State University, USA, Tel +1 301-687- 4299, Fax +1 301-687-3034
Received: July 26, 2021 | Published: August 9, 2021
Citation: Munasinghe KA, Matthews TC, Seddon WL, et al. Characterization of Chicken Skin Collagen Using Liquid Chromatography-Mass Spectrometry (LCMS) and SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE). J Nutr Health Food Eng. 2021;11(1):1-5. DOI: 10.15406/jnhfe.2021.11.00345
Collagen extraction from animal processing wastes has increasingly been of interest due to the abundance of collagen in by-products. Collagen is used extensively in the health care, food and beverage, and cosmetic industries, which has increased the need for alternative collagen sources. The extraction of collagen from chicken by-product creates effective use of underutilized resources and a low-cost production of collagen. The objective of this research was to analyze chicken collagen and compare the characteristics with bovine type I collagen to identify possible methods to use chicken collagen as an alternate source. Chicken skins were obtained from the Frostburg State University cafeteria and pretreated with 0.1N NaOH, 10% butyl alcohol, and 0.1N HCl in the microbiology lab to remove non-collagenous protein, fat, and inorganic material, respectively. Collagen was extracted with 0.5M acetic acid containing pepsin (2g/100g) and precipitated with a 2.6M NaCl solution that had 0.05M tris hydroxymethyl aminomethane. The collagen extract was centrifuged at 20,000g at 4°C for one hour. The precipitate was salted out with 0.5M acetic acid before being dialyzed against distilled water. Collagen extracted from acetic acid and with pepsin from the chicken skin provided the highest collagen yield with P <0.05 for the level of significance. The LC- MS results indicated that the mass spectra for major correlated peaks were similar for chicken skin collagen and bovine type I collagen with similar retention times. The LC-MS results suggested that chicken collagen and bovine collagen type I have similar chemical compositions. The SDS-PAGE page results also confirmed that the chicken skin and bovine type I collagen have similar banding patterns with α-1 and α-2 chains that have 148 kD and 130 kD molecular weights, respectively. These results indicated that the chicken skin collagen was a type I collagen and can be used as an alternate collagen source.
Keywords: Chicken by-products, chicken collagen, acetic acid and pepsin extraction, LC-MS, SDS-PAGE
The waste materials generated by chicken processing can be utilized as an alternate source for collagen to meet high demands of collagen in the future.1 Estimated revenue of collagen in 2027 was 7 billion USD with the highest consumption in the health care, food and beverages, and cosmetics industries.2 The chicken processing industry is one of the fastest growing food industries in the US and annual broiler production was over 50 billion pounds in 2020.3 Mainly chicken wastes have been utilized for pet food manufacturing, and it is of interest to convert accumulating wastes to a high-protein food for human consumption.4 Chicken collagen extraction from chicken skin is a value-added process and income generator for the chicken industry. In addition, there has been much interest in investigating possible means of employing under-utilized animal by-products to decrease wastes.5 Bovine collagen has a 35% substantial market share while the market has increasing demand for non- mammalian collagen sources such as from poultry and marine life.2 The broiler chicken processing industry can contribute significantly for the future demands of collagen over other collagen sources such as bovine, porcine, and marine due to the abundance of waste generated by the poultry industry. Chicken collagen does not have color, or the objectionable odor seen in marine collagen and the cost of production may remain low compared to the other sources of collagen.1 Collagen is the most abundant protein in the extra-cellular matrix of animals and may enable communication with the cells that build those molecules in the regenerative medicine involved in the process of healing tissues after trauma and restore native functions.6 The structure and chemistry of chicken collagen may have more biocompatibility than marine collagen with the native tissue for diverse application such as osteochondral defects, connective tissue and adipose tissue and mammary gland fibrilization due to being extracted from land-based animal.6 More than twenty different types of molecular structures have been found in collagen and each one is encoded by a specific gene that is responsible for their structural and functional differences.7 The collagen triple helix undergoes several hierarchical levels to perform mechanical properties.8 The triple helical structure which is known as tropocollagen is formed inside cells and then excreted to the extracellular space where many of the collagen molecules undergo self-assembly. The type I collagen is the predominant type of collagen and found mainly in skin and bone.9 The triple helix of the type I collagen is a heteropolymer consisting with two α-1 chains and one α-2 chain. The type II collagen is found in cartilaginous tissues and, with proteoglycans, function as shock absorber in joints and vertebrate.7 The type III is found in skin, blood vessels, and internal organs and is a homotrimer that consisted with three α-1 chains.10 The type IV collagen contributes to filtration systems in basement membranes and basal lamina structures. Basement membranes are present in the walls of blood vessels and regulate the movement of oxygen and nutrient out of the circulation and into the tissues while basal lamina in the skin controls the movement of materials in and out of the dermis.7 Traditionally, collagen was extracted from acetic acid, NaOH, and citric acid. It was found that higher yields of collagen can be extracted through partially cleaving molecule with pepsin.11-13 The acetic acid and citric acid extraction of the skins of fresh Baltic Cod was 20%.14 Chicken collagen extraction yields of acetic acid, NaOH,3 and citric acid were about 6% and that of pepsin extraction was over 38%.1 Moreover, it was found that pepsin extracted collagen has better tensile strength.15 Comparison of chicken collagen with bovine collagen will be beneficial in exploring possible applications of chicken collagen as an alternate collagen. LC-MS is one of the techniques that can be utilized to compare organic compounds in collagen extracts. In LC-MS, individual components of the injected samples are separated in the separating column based on their interactions with the stationary phase. Mass to charge ratio of components are determined for comparison with intensity increasing with component concentration.16 The objective of this research was to analyze chicken collagen and compare the characteristics with bovine type I collagen to identify possible methods to use chicken as an alternate source of collagen.
Broiler chicken skins were collected from the Frostburg State University cafeteria and stored in the microbiology lab at 4℃ until they were used for material preparation. The raw material preparation was adopted from Kittiphattanabawon et al.,17 with some modifications. Chicken skins were cut into small pieces using a pair of scissors prior to pretreatment. Non-collagenous proteins were removed by stirring chicken skin with 0.1N NaOH with sample/solvent ratio of 1:6 (w/v) for 6 hours at 4℃, while the NaOH solution was changed every 2 hours. At the end of 6 hours, the sample was washed with distilled water. The deproteinated skin sample was stirred with 10% butanol with sample/solvent ratio of 1:6 for 24 hours while the butanol was changed every 6 hours to remove fat. The defatted sample was washed with distilled water and stirred with 0.1N HCl with sample/solvent ratio of 1:6 (w/v) for 24 hours. The pretreated chicken skin sample was placed on a beaker covered with a double layered cheese cloth and washed with distilled water three times.
Collagen extraction
The method developed by Nalinanon et al,18 was used for the extraction of collagen with some modifications. The pretreated chicken skin was stirred with 0.5M acetic acid containing pepsin (2g pepsin/100g treated chicken skin) with the sample/solvent ratio for 48hours at 4℃. The solution was filtered with a double layered cheese cloth and the filtrate was collected to precipitate collagen. 4 Running Head: Characterization of Chicken Skin Collagen
Collagen precipitation
The extracted collagen was precipitated according to the method described by Kittiphattanabawon et al.,17 The chicken collagen filtrate was mixed with 2.6M NaCl and 0.05M Tris hydroxymethyl aminomethane and centrifuged using a Beckman J2-MC centrifuge (Beckman Coulter Life Sciences, Indianapolis, IN) at 20,000g for 60min at 4℃. The precipitate was salted out with 0.5M acetic acid for 24 hours and washed with distilled water three times. Both wet and freeze-dried chicken collagen were used for further analysis.
Liquid Chromatography-Mass Spectrometry
The Liquid Chromatography-Mass Spectrometry (LC-MS) analysis was performed on a Prominence-i LC2030C 3D Plus (Shimadzu, Columbia, MD) consisting of a degasser, low pressure mixer, pump, temperature controlled autosampler, a column oven, and diode array detector. It was coupled to a LCMS-2020 single-quadrupole instrument (Shimadzu, Columbia, MD). Chromatographic separation was carried out on a Roc C-18 column, 100 X 3mm (Restek, Bellefonte, PA) with 3µm packing with a guard column. A gradient elution method was adapted from Song et.al.,16 The mobile phase consisted of solvent A with 0.1% formic acid in ultrapure water (100:0.1 v/v) and solvent and B with 0.1% formic acid in acetonitrile (100:0.1v/v). A linear gradient with a 1.6ml/min flow rate was started at 5% B and ramped to 40% B from 0-16min, then held at 40% B from 16-40min. The injection volume was 10µl and the column was held at 40℃. Electrospray Ionization (ESI) was used as MS source under the following conditions: Nebulizing gas N2 at 1.5L/min, drying gas (N2) at 15L/min interface at 350℃, desolvation line 250℃, and heat block at 400℃ with voltages automatically optimized by the tuning program. The detector was run in positive ion mode and scanned from 100 to 2000 m/z at a scan speed of 2143 u/sec. The wet and freeze-dried chicken collagen samples were tested along with commercially available bovine type I collagen to compare chemical composition.
SDS-Polyacrylamide Gel Electrophoresis: Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) was performed according to the methods described by Nalinanon et al.18 with some modifications. Sample weights of 0.2mg of wet chicken collagen, freeze dried chicken collagen, and bovine type 1 collagen were dissolved in 100µl of 0.02M Sodium Phosphate buffer prepared by adding 0.56 µl monobasic and 1.44µl of dibasic Sodium Phosphate, 1g SDS (1% w/v), and 21g of urea. Each individual vial was loaded with 10µl of each collagen sample and 10µl of Laemmli sample 5 buffer (Fisher Scientific, Waltham, MA) and heated for 5 min in a Dry Bath Incubator (Fisher Scientific Waltham, MA). SDS-PAGE was performed using Mini-ProteanTGX Stain-free Precast 4-20% Gel (Bio-Rad, Hercules, CA) using a Precision Plus ProteinTM KaleidoscopeTM Prestained (Bio-Rad, Hercules CA) with a 10-250 kD range. The working buffer was made by mixing 100ml concentrated buffer (25mM Tris, 192 mM Glycine, and 0.1% w/v SDS) with 900ml autoclaved distilled water to meet the final concentration of 1X solution. The electrophoresis was done using 150V until the tracking dye reached the bottom of the gel. The gel was stained with Coomassie blue R-250 overnight and distained with distilled water two times. The gel was visualized using an Alpha Innotech AlphaImager (Alpha Innotech, San Leandro, CA). Chicken collagen yields were analyzed using ANOVA at P<0.05 significant level.
Collagen extraction
The chicken skin was cut into small pieces and used for the pretreatments directly without further mincing with a blender. Chicken collagen can be extracted from acetic acid, NaOH, citric acid, one step-acetic acid and one- step pepsin, and two-step acetic acid and one-step pepsin.17,1 A yield comparison study showed that one-step acetic acid and one step pepsin extraction and two- step acid one-step pepsin had higher yields than traditional collagen extraction methods with acetic acid, NaOH, and citric acid. The acetic acid and pepsin (one-step acid and one-step pepsin) extraction was used in this research due to less processing time than two-step acetic acid and one-step pepsin extraction. Nalinanon et al.,18 showed that the pepsin activity during Bigeye Snapper skin collagen extraction was enhanced by the pre-swelling step with acetic acid before the pepsin digestion at 4℃. Collagen extract from the chicken skin was colorless and did not have an objectionable odor.1 Morimura et al.,19 showed that some materials derived from livestock and fishery waste tend to have the odor of their source and would limit the use of those sources without masking the odor with additional processing steps.
LC-MS results analysis
LC-MS results were utilized to compare the major extract component of chicken collagen with bovine type I collagen. All collagen samples, wet and freeze-dried chicken collagen as well as bovine type I collagen samples, were prepared for the LC-MS as shown in the table below (Table 1). Samples were weighed then dissolved in ultrapure 6 water and sonicated. Formic acid was added to samples as needed to assist in dissolution. Samples were filtered through a 0.22µm membrane and held at 4℃ until injection. Data processing was completed in R using the XCMS package from Bioconductor.20-22 Peaks detected in the Base Ion Chromatogram (Figure 1) show three correlated peaks at 312. 358, and 1070 seconds. These results indicated that the chicken collagen and bovine type I collagen have similar components present with similar relative concentrations. Mass spectra from correlated peaks (Figure 2) indicated that both similar m/z peaks and relative abundancies across the three samples. This result further indicates that the chicken collagen samples have the same composition as bovine type I collagen.
Sample |
Mass (g) |
Volume of water(ml) |
% Formic acid |
|
|
|
|
|
|
1. |
Wet chicken collagen |
0.1569 |
10 |
0 |
2. |
Bovine Type I |
0.0016 |
10 |
0.1 |
3. |
Freeze dried chicken collagen |
0.0337 |
10 |
0.1 |
Table 1 Sample preparation for the LC-MS
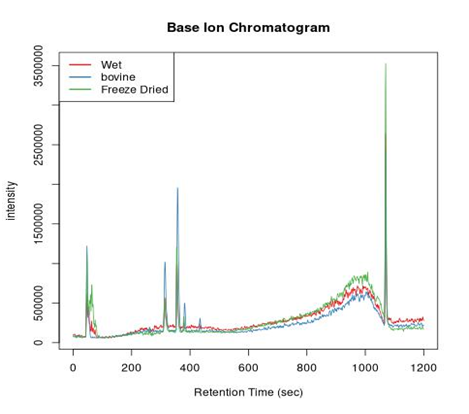
Figure 1 Base Ion Chromatogram: red-wet chicken collagen, blue-bovine type I collagen, and green-freeze-dried chicken collagen.
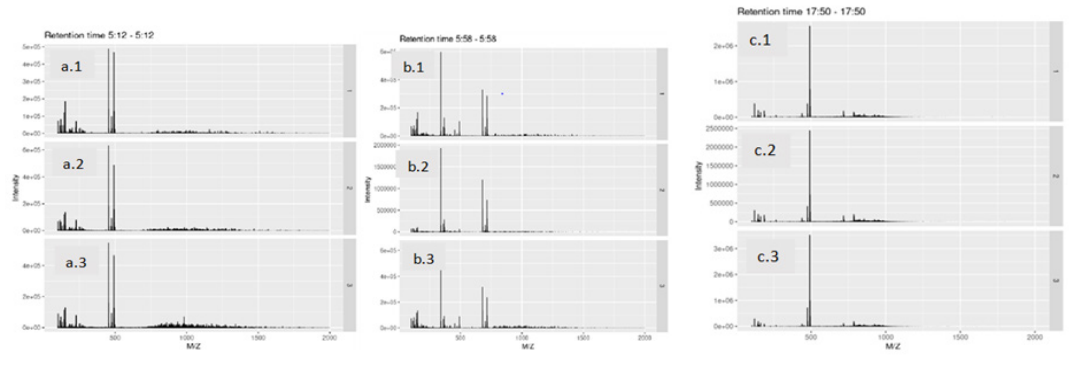
Figure 2 Mass Spectra of correlated peaks at given retention time. a- 312 seconds, b.- 358 seconds, c. 1070 seconds. 1. wet chicken collagen 2. bovine type I collagen 3. freeze-dried chicken collagen (M/Z: mass in atomic mass units to charge ratio).
SDS-PAGE analysis results
The electrophoresis patterns of wet and freeze-dried chicken collagen and bovine type I collagen were analyzed using SDS-PAGE. The results indicated that banding pattern for the chicken collagen had similarities to bovine type I collagen because of having similar subunits. The two bands had been identified as α1 and α2 (Figure 3) and have molecular weights of 149 and 130 kD, respectively. Zhang et al.,23 showed similar banding patterns for type I collagen in fish scales. Nalinanon et al.,18 also showed that there was no difference in relative mobility of high molecular weights between Bigeye Snapper skin and calf skin type I collagen. According to Zhang et al.,23, higher molecular weights (>250 kD) might be ꞵ and ɤ subunits in chicken collagen and they are similar to bovine collagen as well. Satio et al.,24 observed similar results for ꞵ and ɤ bands in Sea Cucumber (Stichopus japonicus). These results indicate that type I collagen is the major collagen found in the chicken skin.10
Chicken waste was used to extract collagen from chicken skin using 0.5M acetic acid and pepsin (2g/100g treated chicken skin). Pretreatment procedures were conducted to remove non-collagenous protein, fat, and minerals from chicken skins. The LC-MS results indicated that there was a major correlation between peaks of bovine type I collagen and chicken skin collagen. Similar mass spectrum patterns and retention times indicated that they have similar chemical composition. The SDS-PAGE results showed that both bovine and chicken collagen shared a molecular weight range between 25-250 kD. The α-1 and α-2 bands of chicken collagen matched with bovine collagen type I. In addition, it is an advantage that chicken collagen is colorless and had no characteristic odor to avoid additional processing steps. Based on the LC-MS and SDS-PAGE results, chicken skin collagen is a type I collagen and can be utilized as an alternate source for bovine type I collagen. This study can conclude that collagen extracted from chicken skin with acetic acid and pepsin can be utilized as a value-added product to meet increasing demand for collagen in the future.
The authors disclose no conflicts.
The authors would like to thank the Faculty Development Committee of Frostburg State University, Maryland for funding this project. The authors also thank Dr. David Puthoff at Frostburg State University for helping them with preparation of solutions for the SDS-PAGE. The authors extend their thanks to Banuja Munasinghe at University of Maryland, College Park for reviewing this paper.

©2021 Munasinghe, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.