Journal of
eISSN: 2373-4310


Research Article Volume 1 Issue 5
1Department of Biotechnology, University of Food Technologies, Bulgaria
2Department of Informatics and Statistics, University of Food Technologies, Bulgaria
3LB Bulgaricum PLC, Bulgaria
Correspondence: Angel Angelov, Department of Biotechnology, University of Food Technologies, 26 Maritza Blvd, Plovdiv 4002, Bulgaria, Tel +35932603608, Fax 0035932643611
Received: July 05, 2014 | Published: September 9, 2014
Citation: Blagoeva G, Milev M, Minkova S. Assessment of lactic acid bacteria and enterobacteriaceae counts in Bulgarian probiotic products by TEMPO® system and ISO methods. J Nutr Health Food Eng. 2014;1(5):192-196. DOI: 10.15406/jnhfe.2014.01.00029
Some issues related to the microbiological quality and safety of probiotic products requires the use of fast and reliable microbiological methods for control throughout production and along the market chain. One of the authenticity/quality issues related to probiotic products at the market is the actual content of viable lactic acid bacteria (LAB) in them. Claims on the labels usually state live LAB content above 106-107cfu/ml or g, but market studies show that in many occasions this may not be true. Another issue related to dairy-based products is their safety, and a common problem is contamination by microorganisms from the Enterobacteriaceae group. The aim of this study was to compare the performance of the alternative MPN-based methods TEMPO LAB® and TEMPO EB® with the reference methods ISO 15214:1998 and ISO 21528-2:2004 for the enumeration of LAB and Enterobacteriaceae in various probiotic products. The study was conducted with 114 Bulgarian probiotic foods and supplements. Results showed good agreement between the TEMPO and the respective ISO methods, which confirms that TEMPO LAB® and TEMPO EB® can effectively be used as rapid alternative methods for assessing the content of live LAB and Enterobacteriaceae as important quality and safety indicators for probiotic products.
Keywords: probiotic foods, quality assessment, method comparison, tempo® lab, tempo® eb
A huge variety of probiotic products is available on the market. Those could be various solid, semi-solid or liquid foods, lyophilized starter cultures or probiotic supplements (capsules or powders). The labels of probiotic products should contain the names of the microbial species (mostly belonging to the group of lactic acid bacteria) associated with the claim “probiotic”, as well as the content of viable cells of those species in the product before the expiry date.
Claims on the labels usually state live LAB content above 106-107cfu/ml or g, but over the past 20years concerns have been raising that in many occasions this may not be true, meaning that the actual content of viable lactic acid bacteria (LAB) in probiotic products is one of the most common quality/authenticity issues associated with them.1
Another important issue is the microbiological safety of these products. The usual media for probiotics production is milk, which is easily contaminated, supporting the growth of various pathogenic bacteria. Therefore, rigorous hygienic precautions and adequate monitoring of the process environment and the finished product should be in place in order to ensure the quality and safety of the final product.
The common microbiological parameters monitored for quality and safety of dairy products are applicable to probiotic products as well. Of these, Enterobacteriaceae is usually tested as a hygiene indicator intended to verify the hygiene programs applied at the production premises.2,3
Microbiological testing for the presence of Enterobacteriaceae is largely applied for dairy products, and some pathogenic species of this family, such as E. sakazakii, represent a significant risk related to powdered milk products. A FAO meeting report4 states that the presence of Enterobacteriaceae in packaged powdered infant formulas is not due to the organisms surviving the process, but rather as a result of recontamination which is due to post-heating processes such as drying, conveying, tipping, mixing with additional ingredients and filling or packaging. Recontamination could also be related to the presence of microorganisms in the processing environment and to equipment that is in contact with the product.5
Dry dairy-based probiotic formulations are of the same nature as powdered milk products in terms of food matrices and production process, and therefore testing for Enterobacteriaceae as a hygienic indicator should be applied for these products as well, with certain exceptions, when a representative of Enterobacteriaceae (e.g. E. coli Nissle 1917) is an intended part of the probiotic formulation.
Usually, conventional standard methods such as standard plate count and most probable number (MPN) are applied in routine microbiological analyses. The most probable number (MPN) technique uses liquid media for detecting and enumerating Enterobacteriaceae, coliforms and E. coli and it is particularly useful when low levels of these bacteria are present. Traditional methods for enumerating lactic acid bacteria in food products are time consuming, involving direct plating on solid selective media prepared by pour or spread plate technique. However, the high number of samples analysed daily require new enumeration methods with improved efficiency, convenience and ease-of-use.
Microbiological analysis is a rapidly developing area, in which “faster” and “more accurate” are key elements in meeting the demands of the food industry, which needs to ensure that product quality and safety are maintained. To meet this increased demand, rapid methods to perform microbiological analysis are constantly being developed, resulting in new time, labour and money-saving technologies, agars and approaches for microorganism detection, enumeration and identification.
Bio Merieux S. A. has designed an automated MPN method, TEMPO®, which standardizes the numerous preparation steps, interpretation, and test results. The outcome is a fast, accurate method which can be used for the enumeration of various target organisms, including Enterobacteriaceae, E. coli, total viable count, lactic acid bacteria and several other, which comprise about 90% of the most commonly performed microbiological analyses of foods.3,6
Rapid and reliable enumeration of viable lactic acid bacteria is very important for the quality/authenticity assessment of probiotic products, and therefore all stakeholders in this field are interested in methods offering a better alternative to the traditional and reference microbiological methods.
Also, with their high levels of lactic acid bacteria, probiotic products represent a challenging analytical matrix for the enumeration of Enterobacteriaceae, which, as contaminating microorganisms, might be in very low numbers in the product and therefore difficult to enumerate reliably. The aim of this study was to compare the performance of the alternative MPN-based methods TEMPO® LAB and TEMPO® EB with the reference methods ISO 15214:19987 and ISO 21528-2:20048 for the enumeration of LAB and Enterobacteriaceae in various probiotic products.
Probiotic products
A total of 114samples from two food categories were analysed in the present study: Group 1 included 59samples of lyophilized probiotic supplements (capsules and powders) and Group 2 included fermented foods - 52 yogurt samples and 7 cheese samples.
Sample preparation
Appropriate dilutions of each food sample (in accordance with the expected levels of tested microorganisms) were prepared with peptone water. Samples were then homogenized in TEMPO® stomacher bags with filters and adequate amounts were used for analyses by TEMPO and the ISO methods.
Enumeration of LAB
LAB content in all samples was enumerated by the TEMPO® LAB method (bioMerieux, Marcy l’Etoile, France) according to supplier’s instructions using the TEMPO automated system. Samples were also analysed by the reference method ISO 15214:1998 Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of mesophilic lactic acid bacteria – Colony count technique at 30°C.7
Enumeration of Enterobacteriaceae
Thirty-two samples (21 of probiotic supplements and 11 cheese samples) were artificially contaminated with E. coli LMG 8223 (ATCC™ 25922) as a representative of the Enterobacteriaceae family. Before that, all samples were sterilized by irradiation and sterility test was performed by the plate count method (Plate Count Agar (Oxoid, Thermo Scientific), 30°C, 72h).
Artificial contamination of the sterilized samples was made at two levels: approximately 500 and 10000 cfu/g. Each of the spiked samples was mixed well shortly before use. Negative control samples not containing E. coli were also analysed. The presence of artificial contamination in the spiked samples and of natural contamination in all tested probiotic samples was assessed by the TEMPO® EB method (bioMerieux, Marcy l’Etoile, France) according to supplier’s instructions and the reference method ISO 21528-2:2004 Microbiology of food and animal feeding stuffs – Horizontal methods for the detection and enumeration of Enterobacteriaceae - Part 2: Colony-count method.8
Statistical analysis
Each sample was analysed in three replicates by each method. For the statistical analysis, decimal logarithms of the results were used. The statistical equivalence of the two method groups (TEMPO® LAB Vs ISO 15214:1998 and TEMPO® EB Vs ISO 21528-2:2004) was analysed by linear regression, bias and T-test for the averages of the methods, i.e. Two-Sample Assuming Equal Variances.
A total of 114 samples from two food categories - probiotic supplements (powders and capsules) and probiotic foods (yogurts and cheeses) were analysed in the present study with the aim to compare the performance of the alternative MPN-based methods TEMPO® LAB and TEMPO® EB with the reference methods ISO 15214:1998 and ISO 21528-2:2004 for the enumeration of LAB and Enterobacteriaceae in these specific dairy-based matrices.
The mean values of LAB counts estimated by TEMPO® LAB and ISO 15214:1998 in the various tested product groups are presented in Table 1. For all tested products LAB counts were above 107cfu/g, and no significant difference was found between the mean counts estimated by the ISO method and TEMPO® LAB. The standard deviations are within 0.18-0.48 logarithmic units per gram, which reveals considerable conformity of the tests. Differences in results between the ISO method and TEMPO® LAB do not exceed 0.28 logarithmic units, which confirm the excellent performance of the TEMPO method.
Table 2 presents the enumeration results obtained by ISO 21528-2:2004 and TEMPO® EB. Results clearly correlated with the two levels of artificial contamination with E. coli LMG 8223, with no significant difference between the counts estimated by the two methods. Of the natural samples tested only 5 were positive for Enterobacteriaceae-3 powdered probiotic supplements and 2 cheeses, which could be attributed to process hygiene.
Further, the obtained data was explored for statistical equivalent between the ISO and the TEMPO methods for the two microbiological parameters analysed. First, by applying Data Analysis of ANOVA for the results from enumeration of LAB in probiotic products by TEMPO® LAB and ISO 15214:1998, high correlation between the two methods was found, with Pearson correlation coefficient R2=0,774. The linear regression curve between the log values of the two sets of data is presented on Figure 1, and regression parameters for result agreement between the methods are shown in Table 3.
No significant bias (0.19) between the two methods was observed, with a P-Value (0,188868) higher than 0.05 in the 91% confidence interval. The confidence interval [-0.15; 0.04] of the log difference of the two sets was calculated with level of confidence 95% (Table 3).
The length of the confidence interval of the log difference values is very small (absolute value 0.19) which shows the insignificant difference between the averages of ISO and TEMPO methods and, therefore, the excellent agreement between these methods.
This conclusion was confirmed in addition by a statistical ANOVA T-test of Two-Sample Assuming Equal Variances for the averages of the ISO and TEMPO® method. In order to check the statistical difference between the two variances of both methods, the F-Test Two-Sample for Variances was performed on ANOVA. The results prove that there is no statistical difference between the two methods TEMPO® LAB and ISO 15214:1998 at 95% confidence level.
Figure 1 shows close distance (1 logarithmic unit) of two data sets, with all values falling within the range of the two side lines, which again proves the conformity between insignificant difference between TEMPO® LAB and ISO 15214:1998 applied on dairy-based matrices. Previous studies comparing TEMPO® LAB with other LAB enumeration methods (PetrifilmTM and ISO 15214) applied to different food matrices also showed good agreement between the methods.9,10
BioMerieux has obtained AFNOR validation for the TEMPO technique as an alternative analytical method to ISO 21528-2:2004 for Enterobacteriaceae. The TEMPO® EB test was validated also and certified as a Performance-Tested Method (PTM) by the AOAC Research Institute as an effective method for the enumeration of Enterobacteriaceae in a variety of foods, including raw ground pork, fresh ground beef, heat-processed cooked roast beef, fresh ground chicken, frozen cooked chicken, heat-processed grilled chicken, frozen catfish, heat-processed frozen fish, raw cod, bagged salad, frozen green beans, hash brown potatoes, vanilla ice cream, pasteurized milk, milk powder, pasteurized eggs, rice, and dry pet food.11,12
In the present study, enumeration of Enterobacteriaceae was performed for artificially and naturally contaminated samples of probiotic supplements and cheeses. Regression analysis of results obtained by the two methods showed linear correlation (Figure 2).
The correlation coefficient of the two methods is R2=0,755. The P-value and corresponding confidence interval between the two methods indicate that there is no significant bias between the TEMPO® EB and the reference method (Table 4).
The F-Test Two-Sample for Variances on ANOVA applied on the two variances of the methods confirmed that there is no significant difference between TEMPO® EB and ISO 21528-2:2004 method (95% confidence interval).
Previous comparison studies of TEMPO® system for examinations of different microbiological parameters (total viable count, coliforms, E. coli count) in various food matrices also showed high correlation with the respective reference methods.2,3,13–15 As an example, Zitz et al.,16 evaluated three TEMPO® applications to enumerate total viable count, total coliforms and E. coli in milk and soft fresh cheeses and found that the TEMPO® methods showed equal or better precision and, for some parameters, higher recovery compared to the traditional MPN-based methods.
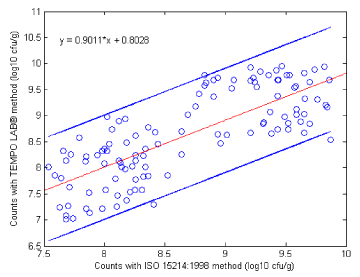
Figure 1 Linear regression curve for the enumeration of lactic acid bacteria in probiotic foods by TEMPO® LAB and ISO 15214:1998.

Figure 2 Linear regression curve for the enumeration of Enterobacteriaceae in probiotic foods by TEMPO® EB and ISO 21528-2:2004.
Sample Type |
No. of Samples |
Mean Counts LAB, log cfu/g |
|
|---|---|---|---|
ISO 15214:1998 |
TEMPO® LAB |
||
Probiotic Supplements (Capsules) |
24 |
8.12±0.24 |
8.34±0.20 |
Probiotic Supplements (Powders) |
35 |
7.88±0.18 |
7.62±0.12 |
Cheeses |
7 |
8.94±0.48 |
9.14±0.37 |
Yogurts |
48 |
8.36±0.42 |
8.08±0.26 |
Table 1 Enumeration of LAB in probiotic products by TEMPO® LAB and ISO 15214:1998
LAB, lactic acid bacteria; log, logarithm; cfu, colony-forming units
Sample Type |
No. of Samples |
Mean counts Enterobacteriaceae log cfu/g |
|
|---|---|---|---|
ISO 21528-2:2004 |
TEMPO® EB |
||
Probiotic Supplements |
21 |
2.78±0.151 |
2.59±0.231 |
Cheeses |
11 |
2.17±0.141 |
2.02±0.161 |
Natural Samples |
5 |
1.13±0.29 |
1.24±0.42 |
Table 2 Enumeration of Enterobacteriaceae in artificially and naturally contaminated probiotic products by TEMPO® EB and ISO 21528-2:2004
1Low level of artificial contamination with E. coli 500cfu/g)~LMG 8223 (
2High level of artificial contamination with E. coli 10000cfu/g)~LMG 8223 (
Results represent the mean of triple analyses of all samples within a product group. For natural samples only are means of the 5 Enterobacteriaceae-positive samples are presented.
EB, enterobacteriaceae; log, logarithm; cfu, colony-forming units
|
Bias |
Confidence interval |
P-value |
TEMPO® LAB Vs ISO 15214:1998 |
0.19 |
[-0.15; 0.04] |
0.189 |
Table 3 Evaluation of agreement between TEMPO® LAB and ISO 15214:1998
LAB, lactic acid bacteria; Vs, versus
|
Bias |
Confidence Interval |
P-value |
TEMPO® EB Vs ISO 21528-2:2004 |
0.20 |
[-0.12; 0.08] |
0.004 |
Table 4 Evaluation of agreement between TEMPO® EB and ISO 21528-2:2004
EB, enterobacteriaceae; Vs, versus
The performance of the automated TEMPO® methods for the enumeration of LAB and Enterobacteriaceae proved to be statistically equivalent to the respective standard methods for these microorganism groups. By ensuring several ten-fold dilution steps of high precision within a single manipulation, and by eliminating media preparation, manual counting and visual interpretation of results, TEMPO® LAB and TEMPO® EB are not only easier to use and less time-consuming, but also provide rapid and reliable results, increased efficiency and labor-saving. This study confirmed that the TEMPO® LAB and TEMPO® EB offer an efficient rapid method alternative to ISO 15214:1998 and ISO 21528-2:2004, respectively, for the enumeration of LAB and Enterobacteriaceae in various probiotic products.
The present work was accomplished under project “Functional probiotic foods – the contribution of modern agricultural science to food technologies” (ProBio) funded by America for Bulgaria Foundation.
The author declares no conflict of interest.

©2014 Blagoeva, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.