Journal of
eISSN: 2377-4312


Case Report Volume 9 Issue 4
1Veterinary Clinics Department, State University of Londrina, Parana, Brazil
2College Qualittas, Padre Leonel França Street, São Paulo, Brazil
3Veterinary Clinicand Surgery Department, Federal Universityof Minas Gerais, Brazil
Correspondence: Mauro José Lahm Cardoso, Professor at College Qualittas, Padre Leonel França Street, 641, Jardim Leonor, Campinas, São Paulo, Brazil
Received: August 12, 2020 | Published: August 19, 2020
Citation: Rivas BB, Cardoso MJL, Cecci GRM, et al. Primary hyperaldosteronism in a cat. J Dairy Vet Anim Resv. 2020;9(4):113-117. DOI: 10.15406/jdvar.2020.09.00290
A feline, male, 12 years of age, mixed breed was attended at the Veterinary Hospital of the State University of Londrina, showing apathy, paresis, cervical ventroflexion, sialorrhea, mydriasis and blindness. Complementary exams showed azotemia, hypokalemia and a unilateral adrenal nodule. The measurement of serum aldosterone confirmed the diagnosis of hyperaldosteronism caused by adrenal adenoma. After three months of oral medication, a surgical excision of the adrenal gland was performed and, 72 hours later, the patient died of renal complications. This study highlights the importance of considering primary hyperaldosteronism as a relevant differential in cats with hypokalemia and hypertension.
Keywords: adenoma, aldosterone, adrenal gland, hypertension, hypokalemia
Primary hyperaldosteronism (PAH), also known as Conn's disease, is a rare endocrine disorders that is characterized by excessive aldosterone secretion from the glomerulus area of the adrenal gland.1 This syndrome was first described in Human populations in 1955 and in 1983, the first report of the disease was published in domestic cats.2 The most common clinical signs involve muscle weakness secondary to hypokalemia and ocular alterations related to systemic arterial hypertension.3 The causes of PAH include adrenal neoplasia, unilateral or bilateral, and idiopathic adrenal hyperplasia, similar to what occurs in Human populations.4 In cats, adenomas and carcinomas are most commonly observed.2 Some of these tumors, especially carcinomas, in addition to secreting mineralocorticoids such as aldosterone, also secrete glucocorticoids and sex steroids.4 There has even been a report of hyperprogesteronism associated with diabetes mellitus, with concomitant hyperaldosteronism.5,6 Although PAH is a disease usually found in middle-aged to elderly cats,1 it has been described in a 5-year-old feline.2 To date there is no indication of a predilection for breed or sex,1,7 however in all reports the animals were castrated.4
The disease manifests itself mainly through hypocalcemic myopathy and/or sudden onset blindness, in addition to other clinical manifestations associated with marked hypertension, such as edema, hemorrhages, and central nervous manifestations. Clinical signs such as polyuria, polydipsia, polyphagia, secondary diabetes mellitus, poor condition of the pelvis, bulging abdomen, and thin, friable skin, due to excessive secretion of other hormones, such as cortisol and progesterone, may occur.4 Diagnostic methods include measurement of systemic blood pressure (SBP), biochemical and electrolytic profiling of the patient (increased creatinine and urea may occur), aldosterone measurement, imaging tests, and histopathological examination. In Human populations, the aldosterone: renin (ARR) ratio is the most reliable screening test to differentiate PAH from secondary hyperaldosteronism, where the increase in the ARR ratio is indicative of PAH. The use of the test was reported in 11 cats, where it aided in the diagnosis of non-tumor PAH.4 Mineralocorticoid function tests (MFT) are still being studied in cats.8
Conservative treatment consists of controlling hypokalemia through potassium replacement and decreasing hypertension with the use of calcium channel inhibitors or potassium-sparing diuretics.7,9 The majority of patients demonstrate a good response to conservative therapy, although it is possible to opt for surgical removal of the affected adrenal gland in cases of hyperplasia or unilateral adrenal neoplasia. In this situation, surgical treatment is curative, although the procedure is associated with high mortality rates.4,7,10,11
A 12-year-old feline male, castrated, mixed breed was presented at the Veterinary Hospital of the State University of Londrina (Brazil) showing clinical signs of apathy, paresis, cervical ventroflexion, and sialorrhea. The physical examination identified paresis, plantigrade and ventroflexion, bilateral mydriasis, and blindness due to partial retinal detachment. Systemic blood pressure (SBP) was measured using the oscillometric method, and demonstrated hypertension (220mmHg). The other parameters of the physical examination were within the normal ranges. Complete blood count, biochemical and electrolyte profile were requested (Table 1), showing severe hypokalemia (2.6m mEq/L) and azotemia. There were no significant alterations in other parameters. Thus, was opted for hospitalization for intravenous potassium replacement, SBP monitoring and azotemia control.
Parameters |
Hematological values |
|
Parameter |
|
Reference Values |
RBC count (x106 /uL) |
8,58 |
5.92-9.93 |
Haemoglobin (g/dL) |
13.7 |
9.3-15.9 |
Hematocrit (%) |
39.7 |
29-48 |
Mean corpuscular volume (fL) |
46.3 |
37-61 |
Mean corpuscular haemoglobin |
16 |
11-21 |
Mean corpuscular haemoglobin concentration (%) |
34.5 |
30-38 |
WBC count (/u±) |
12,450 |
3-14,8 |
Segmented neutrophils |
6,890 |
2,500-8,500 |
Band neutrophils |
0 |
0-150 |
Lymphocytes |
4,350 |
1,200-8,000 |
Monocytes |
630 |
0-600 |
Eosinophils |
580 |
0-1,000 |
Basophils |
0 |
0-150 |
Platelet count (x103 /uL) |
540 |
200-600 |
|
Biochemical values |
|
ALT (U/L |
178 |
10-100 |
Albumin (g/dL) |
4.1 |
2.5-3.9 |
Albumin/globulin ratio) |
1,5 |
0.35-1.5 |
Alkaline phosphatase (U/L) |
93 |
10-50 |
BUN (mg/dL) |
159 |
14-36 |
BUN/creatinine ratio |
46.7 |
4-33 |
Calcium (mg/dL) |
9.3 |
8.2-10.8 |
Chloride (mEq/L) |
114 |
104-128 |
Cholesterol (mg/dL) |
156 |
75-220 |
Creatinine (mg/dL) |
3.4 |
0.6-2.4 |
Globulin (g/dL) |
2.7 |
2,3-5,3 |
GGT (U/L) |
11 |
1-10 |
Glucose (mg/dL) |
172 |
70-170 |
Phosphorus (mg/dL) |
5.6 |
2.4-8,2 |
Potassium (mEq/L) |
2.6 |
3.4-5.6 |
Sodium (mEq/L) |
174 |
145-158 |
Triglycerides (mg/dL) |
72 |
25-160 |
Table 1 Hematological and biochemical values of the feline patient, 12 years old with a diagnosis of hyperaldosteronism
The cat was submitted to more detailed diagnostic study, with urinalysis and urine protein/creatinine ratio(PCR), test for feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV), echocardiography and abdominal ultrasound. During the abdominal ultrasound and radiography (Figure 1A&B), an important increase in the left adrenal was identified, with approximate measurements of 2.5 x 3.1 cm in diameter, without invasion to adjacent structures and organs. The treatment consisted of fluid therapy and intravenous potassium replacement, as well as antihypertensive therapy with oral calcium channel blocker (Amlodipine 0.3 mg/kg every 12 hours). After normalization of serum levels and remission of clinical signs of hypokalemic polymyopathy and hypertension, the patient was discharged for conservative treatment at home with calcium channel blocker (Anlodipine 0.3 mg/ kg every 12 hours), potassium-sparing diuretic (Spironolactone 1 mg/kg every 12hours) and oral potassium citrate replacement (500 mg every 24 hours).
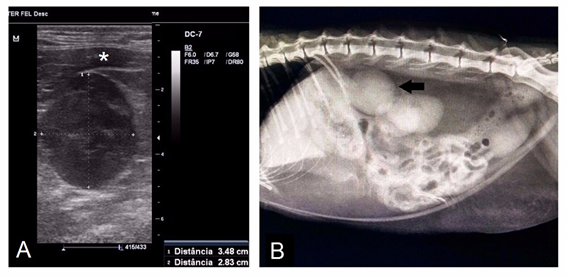
Figure 1 Diagnosis by adrenal mass imaging. A: Ultrasonographic image of the left adrenal gland, demonstrating a significant increase and closely linked to the V.cava caudalis (indicated by the asterisk). B: Abdominal radiography of the patient in lateral projection, the arrow indicates the adrenal gland.
When the patient returned, the general clinical framework was reevaluated and biological material was collected for serum aldosterone measurement by the radioimmunoassay method. The patient presented a good general condition and the serum potassium (4.5 mEq/L) and SBP (132 mmHg) were within the normal ranges. The result of the hormonal dosage of aldosterone was 516.36 pg/ml (reference value 70.0-140.0 pg/ml), confirming the suspicion of PAH. The patient remained stable with oral medication for three months, after which, surgical excision was performed, due to the progressive growth of the mass and difficulty in drug administration. A left adrenalectomy was performed using celiotomy technique. The adrenal gland was increased (Figure 2A), irregular in appearance, neovascularized, and adhered to the abdominal wall. During the surgical procedure, there was bleeding and significant periods of hypotension. After surgery, a whole blood transfusion was performed and the SBP remained stable, but the patient died on the third postoperative day due to acute renal failure, hyperkalemia, and azotemia/uremia.
The left adrenal gland was 4.5 cm in diameter, with a firm consistency alternating with soft, multifocal areas, with a multilobular aspect and totally encapsulated (Figure 2A). When cut, it had a lobular aspect, yellowish in color, with reddish multifocal areas, delimited by connective tissue, which completely replaced the adrenal gland parenchyma. Additionally, there were multiple cysts, distributed multifocally, with diameters from 0.1 to 0.3 cm. Histologically, normal gland architecture was replaced by densely cellular, expansive, and encapsulated neoformation, composed of well-differentiated cells, with characteristics of cortical cells, predominantly arranged in compact trabeculae (Figure 2B). The cells were polygonal, with defined borders, cytoplasm ranging from clear eosinophilic to vacuolated, sometimes completely lipidized, round nuclei with a visible nucleolus, and generally marginalized chromatin (Figure 2C). Areas of necrosis, delimited by neutrophils, were present (Figure 2D). Scarce mitotic figures and few binucleated cells were observed.
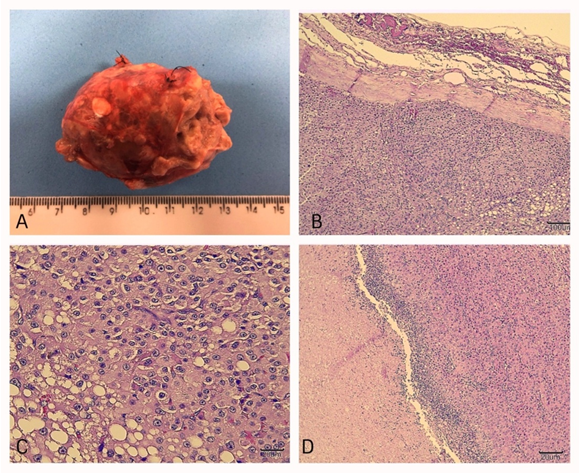
Figure 2 Images of the macroscopic and microscopic evaluation of the removed adrenal gland. A: left adrenal gland measuring approximately 4.5cm in diameter. B: Microscopic image 10x magnification. Replacement of the normal parenchyma with densely cellular, expansive, encapsulated neoformation, with a predominance of cells arranged in trabeculae. C: Microscopic image, 40x magnification. Polygonal cells with well-defined cytoplasmic limits can be observed; abundant cytoplasm, slightly eosinophilic, sometimes vacuolated and filled with lipid material. D: Microscopic image, 10x magnification. An area of necrosis delimited by a neutrophilic inflammatory infiltrate can be observed on the left, in contact with the neoformation on the right.
Primary hyperaldosteronism was suspected due to the presence of hypokalemic neuropathy associated with severe arterial hypertension and the presence of a palpable mass in the abdomen. The presence of a mass on the adrenal gland during the ultrasound examination contributed to this suspicion. However, the definitive diagnosis was only confirmed with the measurement of serum aldosterone. Other causes may be associated with severe hypokalemia and hypertension such as chronic kidney disease.12 In addition, hypertension can aggravate a pre-existing kidney injury. The patient presented signs of chronic nephropathy on the abdominal ultrasound and also presented azotemia, which may suggest that hypokalemia and hypertension were caused by excess aldosterone as well as by kidney damage. Hypokalemic neuropathy differs from diabetic neuropathy, which was ruled out due to the absence of hyperglycemia and glycosuria.
The patient's diet, gastrointestinal loss, use of diuretics, hyperthyroidism, primary or secondary hyperaldosteronism, or even urethral clearance are other possible causes of hypokalemia and were ruled out. Secondary disease usually develops in domestic cats as a physiological response to the stimulation of the renin-angiotensin-aldosterone system, and can be observed in animals which are dehydrated, hypotensive, cardiopathic, and have liver disease, or chronic kidney disease.1,13 To differentiate between primary and secondary hyperaldosteronism, in the absence of adrenal neoplasia, it is necessary to measure renin and aldosterone and determine the aldosterone: renin ratio; if this is decreased, it indicates secondary origin. The measurement of renin was not performed in the current case due to the high cost and lack of reliable available tests in Brazil.
Sudden blindness is also one of the main clinical manifestations of the disease, which is secondary to a hypertensive framework. In cats with PAH that present an increase in SBP, hyphema and retinopathy or choroidopathy are common, and the condition may progress to retinal detachment.2 Mydriasis and partial retinal detachment were observed in the patient, with hypermetria when walking and the animal bumping into objects. According to reference14 other side effects of constant hypertension are persistent kidney damage and cardiac hypertrophy, both ruled out in the patient. The alterations found in the routine laboratory tests include mainly hypokalemia, hypernatremia, and elevated urea and creatinine levels.15,16 Hypokalemia and azotemia were also observed in the feline patient reported here, however the sodium dosage was not elevated.
Conservative treatment of the disease consists of controlling hypokalemia and/or hypertension, performed with oral potassium supplementation, associated with a calcium channel blocker, preferably amlodipine.7 The associated use of spironolactone to control hypertension and hypokalemia usually presents excellent results, since this medication, a potassium-sparing diuretic, is a competitive aldosterone antagonist,10 which was observed in the present report. Although adrenalectomy is the therapy of choice for treatment in unilateral secreting neoplastic masses, complications can arise, such as bleeding resulting from dissection of adhesions, as well as which infiltrations and arrhythmias and hypotension increase morbidity and mortality.4,7,11,15 The feline in this report presented great blood loss during the operation, which resulted in the need for a transfusion in the postoperative period and the blood loss associated with the surgical and anesthetic procedure led to hypotension. This probably contributed to the development of ARI that led to death. Adrenalectomy results in healing in approximately 70% of patients with PAH.17 However, complications in the trans and postoperative period contributed to death, as well as pre-existing chronic kidney disease (CKD). The long-term use of spironolactone may have blocked the renin-angiotensin-aldosterone axis and contributed to hypotension and the development of postoperative hyperkalemia. According to reference,17 the most common tumor in cats with PAH is adrenal carcinoma, different from that observed in the current report, since histopathology revealed an adrenocortical adenoma.
It is hoped that the current report will contribute to the differential diagnosis of hypokalemia and hypertension in felines and enable veterinarians to place this disease through differential diagnoses, since many patients die from CKD, which can be a consequence of PAH.
None.
Author declares that there are no conflicts of interest.

©2020 Rivas, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.