Journal of
eISSN: 2377-4312


Short Communication Volume 6 Issue 3
1Vetrepharm Research Inc, USA
2Sheldon Associates, USA
3Nova Vive Inc, Canada
4Scientific Veterinary Institute Novi Sad, Serbia
5Faculty of Veterinary Medicine, University of Belgrade, Serbia
6Faculty of Ecological Agriculture, Educons University, Serbia
Correspondence: Aleksandar Masic, Associate Professor, Faculty of Ecological Agriculture, EDUCONS University, Vojvode Putnika 87, Sremska Kamenica 21208, Serbia, Tel 318 (021)4893610
Received: October 12, 2017 | Published: December 28, 2017
Citation: Nosky B, Biwer J, Alkemade S, Prunic B, Milovanovic A, et al. (2017) Effect of a Non-Specific Immune Stimulant (Amplimune™) on the Health and Production of Light Feedlot Calves. J Dairy Vet Anim Res 6(3): 00179. DOI: 10.15406/jdvar.2017.06.00179
An increasing concern from both producers and consumers regarding the overuse and misuse of antibiotics leading to the development of antibiotic-resistant pathogens dictates the need for new research objectives. These new objectives should be directed towards exploring different management practices and the use of antibiotic alternative products to treat or prevent disease in cattle and other food producing animals. The main objective of this pilot study was to determine if a bovine immunotherapeutic (Amplimune™) derived from Mycobacterium cell wall fraction (MCWF) decreases the incidence and severity of clinical conditions that require antimicrobial treatments, thus reducing the cost of treatment and improving weight gain in young calves entering the feedlot. Four hundred and eighty (480) Holstein steer calves were transported from a large commercial ranch in New Mexico to a feedlot in California. Twenty-four hours following arrival, calves were randomly assigned to one of three treatment groups (control, MCWF 1 and MCWF 3) and allocated to feedlot pens. Animals in MCWF 1 and MCWF 3 groups received either 1mL or 3mL of Amplimune™ subcutaneously, while the animals in the control group were untreated. For the purpose of the study, metaphylactic use of antibiotics was omitted at the time of arrival. Animals were monitored for 102 days and information on the health status, clinical conditions, treatments and body weights were collected and subjected to further analysis. Groups that received Amplimune™ had 13 and 39 less treated animals compared to controls, resulting in calculated savings of USD 535.84 and USD 1021.36 for the MCWF 1 and MCWF 3 groups respectively. In addition, calves treated with Amplimune™ gained a total of 580lbs. and 1,630lbs. more. Overall, there was a significant beneficial effect observed with regards to morbidity, average weight gain, and medical cost per head for both MCWF groups when compared to controls. Results from this pilot study suggest Amplimune™ as a substitute for antibiotic prophylactic treatment in calves entering feedlot.
Keywords: amplimune™, antibiotic, calves, prophylactic
High levels of morbidity and mortality in both dairy and beef calves represent a significant welfare issue and are major sources of economic losses in the cattle industry. The incidence of total feedlot morbidity can reach up to 70% with most being between 15% and 45%. The mortality rate at the same time can reach as high as 15% with most reports indicating an incidence of 1% to 5%.1 Bovine respiratory disease complex (BRDC) is the main reason for morbidity and mortality in feedlot calves.2 The peak incidence of BRDC was observed within the first three weeks after the arrival of calves in the feedlots.1,3 Direct costs are associated with both BRDC morbidity and mortality rates include death loss, treatment, labor and prevention costs, while indirect costs are associated with decreased growth performance and feed efficiency, increased days on feed and decreased carcass quality and market value. Stressful conditions, such as transportation, processing, crowding, temperature extremes or concurrent viral infection can significantly contribute to the higher incidence of morbidity and mortality in young calves.4
Best management practices for newly weaned calves vary, depending on a multitude of factors, including the season of the year when calves are purchased, calf genetics, length of time in the marketing and transportation channels, previous management and vaccination programs, and other factors.3 A traditional preventative measure used to reduce morbidity and mortality on calf operations entering feedlots is prophylactic or metaphylactic administration of antimicrobials on arrival.5 As a potential result of the abundant use of antimicrobials in the cattle industry, high levels of antimicrobial-resistant bacteria have been detected.6There has also been an emergence of livestock-associated methicillin-resistant Staphylococcus aureus in a significant number of Dutch and Belgian calf facilities.7,8 With antimicrobial resistance becoming of greater concern from both an animal and public health point of view, it is imperative to determine strategies to reduce antimicrobial use and resistance without comprising animal welfare. The application of antibiotic alternatives such as immunostimulants can potentially be used to assist the immune system to overcome the immunosuppressive effects of stress and exposure to infectious agents and, consequently, decrease the incidence of morbidity and mortality, as well as the amount of antibiotics used to treat calves.9 Mycobacterium cell wall fraction (MCWF) is a non-specific, biological immune stimulant derived from the soil-borne, non-pathogenic Mycobacterium phlei and is capable of eliciting both innate and cell-mediated immune responses (CMI).10
The bovine MCWF formulation is currently licensed in United States and Canada under the trade named Amplimune™, formerly Immunoboost® (NovaVive US., Athens, USA), as an immunotherapeutic that enhances the immune system to reduce death loss and clinical signs associated with Escherichia coli K99+ induced diarrhea in calves. MCWF also has potential for use in other veterinary applications, such as the treatment of infectious diseases11‒13 and the treatment of cancer.10,14 The mode of action of MCWF is based on the activation of innate and adaptive immunity15 with the purpose of the recognition of, reaction to, and quick recovery from infections.10,11,13 Previous studies have demonstrated that subcutaneous administration of MCWF in day-old calves resulted in decreased morbidity, decreased treatment costs and increased average daily gain.16 In addition, advanced immune maturation as a result of MCWF therapy in calves less than 24hours of age has been demonstrated.17 The current pilot study was designed to test the prophylactic potential of MCWF (Amplimune™) following a single subcutaneous administration of either 1mL or 3mL to light feedlot steer calves, and the resulting impact on health, performance and overall economics.
Description of test animals
Four hundred and eighty (480) clinically healthy Holstein steer calves were sourced from a large commercial calf ranch in New Mexico and transported to a southern California feedlot. Calves were shipped 18hours by commercial truck to the final destination, unloaded from the trucks into one pen on arrival and subjected to veterinary physical examination. The following day, calves were randomly assigned into one of five pens. All calves received routine processing, including two all-flex ear tags marked with a unique identification number, regular vaccination protocol (Once PMH, Merck Animal Health; INFORCE3, Zoetis, USA), vitamin ADE injection and endectocide treatment. For the purpose of this study, metaphylactic antibiotic injections were omitted from routine farm procedures. Animals were individually weighed and the average weight of calves at the time of arrival was 253 pounds. All animals were randomly assigned to their treatment regimen and allocated to assigned pens. Pen A consisted of calves that received no MCWF treatment (Control Group; N=96). Animals in Pen B were administered 1 mL of MCWF (MCWF 1; N=96) and animals in Pen C received 3mL of MCWF (MCWF 3; N=96). Pens D and E each contained 96 animals by including 32 animals of each of the Controls, the MCWF 1 and MCWF 3; with a total of 182 animals commingling in two pens (Table 1).
Mycobacterium cell wall fraction (MCWF)
MCWF (Serial Number 910707A) was manufactured according to the current outline of production filed with the USDA and marketed in the U.S. and Canada under the registered trade name, Amplimune™. MCWF was administered 24hours post-arrival, subcutaneously in front of the shoulder in the volume of 1mL or 3mL according to the corresponding groups.
Study procedures and outcome variables
Calves were evaluated twice daily for any respiratory or gastrointestinal clinical signs for the duration of the study (102 days in the feedlot; September-December). Appropriate therapeutic treatments including antibiotics were administered as directed by a veterinarian in accordance with calf ranch management throughout the trial. At study day 32, individual calf weights, health status and treatment data were recorded. All calves were re-vaccinated with a multi-valent IBR/PI3/BRSV (INFORCE-3, Zoetis, USA) vaccine, a Clostridial 7-way bacterin (Boehringer Ingelheim, Vetmedica, St. Joseph, MO) and were de-liced (CyLence®; Bayer Animal Health, Germany). At day 102 on feed, morbidity, mortality, in-weight, out-weight and health records, including type of treatment and costs, as well as average daily gain, were compiled and analyzed. All medications administered and dates of treatment were noted. Analyses were performed to determine average treatment costs and economic advantages between experimental groups. Weights were recorded in pounds (lbs.) and costs in US dollars (USD).
Statistical analysis
One-way analysis of variance18 and Chi-squared (Chi2) statistical methods were used to determine if there were significant differences between the experimental groups. Data analyzed included comparison of morbidity, mortality, total and average cost of treatments, average weight gain and average daily gain. Data from dead animals were not used for calculation of weight gain and average daily gain.
Pen |
Group |
Number of Calves |
MCWF (Amplimune™) Dose |
A |
Controls |
96 |
0 (Untreated) |
B |
MCWF 1 |
96 |
1 mL |
C |
MCWF 3 |
96 |
3 mL |
D |
D (Commingled) |
(32, 32, 32,) 96 |
0, 1 and 3 mL |
E |
E (Commingled) |
(32, 32, 32,) 96 |
0, 1 and 3 mL |
Table 1 Experimental group assignments
The effects of prophylactic administration of a single subcutaneous dose of a non-specific MCWF immune stimulant (Amplimune™) to Holstein steers was evaluated in a clinical feedlot field study. Calves receiving subcutaneous administration of 1mL or 3mL MCWF were compared to control calves receiving no MCWF. Morbidity, mortality, treatment costs and performance were evaluated. The trial design addressed pen-to-pen treatments and the effect of commingling treatment groups within pens. In general, management practice would have included routine metaphylactic antibiotics used on these types of long-haul light calves, however, for the purpose of this trial, no antibiotics were used on arrival. Antibiotics were only used later in the study to treat clinically sick animals.
Data analysis from individual pens at the end of the study demonstrated that both MCFW 1 and MCWF 3 groups had a significant decrease in morbidity (24% and 62% respectively; p<0.05) compared to control calves (Figure 1). Additionally, the MCWF 3 group had a 44% decrease in number of pulls compared to the MCWF 1 group (p<0.05). Furthermore, the mortality rate was also reduced in the MCWF groups compared to controls (Figure 2), but did not result in statistical significance due to an overall low mortality rate. Analysis of the treatment costs revealed that calves receiving 1mL or 3mL of MCWF by subcutaneous administration on arrival had an average treatment cost of USD 7.92 and USD 4.54 respectively, while treatment costs in the control group were USD 13.03 (Figure 3). This represented a 66% decrease in total medical costs in the MCWF 3 group compared to controls (p<0.05) (Figure 4). Calves in both MCWF groups required fewer treatment days and less antibiotic use compared to control calves, resulting in a 62% less morbidity and 54% decrease in treatment costs (Controls compared to 3mL) (Figure 4). Calves receiving 3mL of MCWF by subcutaneous administration on arrival gained an average of 7.4 pounds more than control calves over the 102day feeding period (Figure 5). This represented a 0.11pound increase in ADG (Figure 6). Overall, calves receiving 3mL MCWF by subcutaneous administration on arrival demonstrated superior health performance than calves receiving only 1ml or no MCWF. Overall, Amplimune™ treated calves had significantly less morbidity and less antibiotic cost than control calves (Table 2).
Data analysis from two commingled pens revealed that the animals receiving 3mL of MCWF demonstrated better health performance compared to both the MCWF 1 and control groups. This was significantly apparent in the number of sick animals (p<0.05) and average cost of treatments (Figure 1) (Figure 4). In addition, no mortality was observed in calves receiving 3mL of MCWF when commingled with other two groups (control and MCWF 1) (Figure 2). Surprisingly, the administration of 1mL of MCWF to 253lb. calves commingled with other calves showed no difference in terms of morbidity, mortality or total treatments compared to controls, and did not show any beneficial effect such as was observed in the MCWF 1 pen (Figure 1). The lack of efficacy for the administration of 1mL of MCWF in commingled pens could be attributed to lower antigen exposure and shorter immune stimulation which was not sufficient to provide prophylactic beneficial effect beyond the first 10days following MCWF administration. In addition, one may assume that the incubation period for BRDC causative agents were longer than the proposed time of immune stimulation with only 1mL of product, thus resulting in similar morbidity.
The rationale for the profound MCWF effect on health and performance when administered to light calves involves several factors for consideration, such as: the status of the calf’s immune system, the immunological effect that MCWF has on the cellular level, clinical and subclinical presence of BRDC, stress and energy utilization. Different compounds able to modulate immune responses by activation or suppression are classified as immunomodulators. MCWF contains multiple immunomodulating compounds, such as trehalose 6,6′-dimycolate (TDM) and muramyl dipeptide (MDP).19 It has been demonstrated that MDP enhances the expression of surface markers on microorganisms that are necessary for cell adhesion and antigen presentation, leading to increased phagocytosis, antimicrobial activity and antibody-mediated cytotoxicity.20 Furthermore, MDP can induce immune responses by triggering production of interferon gamma (IFN-γ) and other cytokines, thus stimulating the activation of lymphocytes.21 TDM is a glycolipid commonly found in the mycobacterial cell wall that is responsible for a non-specific immunomodulation in anti-cancer activity and against infectious diseases.22,23 TDM also can stimulate macrophage activity24 and increase the production of interleukin 6 (IL-6).25,26 It is anticipated that MCWF targets the bovine innate immune and cell-mediated immune responses, providing increased resistance to early infection and reduction of associated clinical signs and providing better energy utilization, resulting in improved food conversion and weight gain. Previously, we demonstrated that MCWF has the ability to attract macrophages and neutrophils and initiate cytokine production in neonatal calves following intravenous, subcutaneous and intramuscular administration (Internal report #98-02SK; manuscript in preparation). Data from the same study also suggested that a single MCWF administration is sufficient to trigger strong innate and cell-mediated immune responses, as measured by IFN-γ, interleukin 2 (IL-2), neutrophil influx and activation of naïve CD4+ and CD8+ cells. In addition, MCWF is able to increase phagocytic and oxidative burst activity following intrauterine administration in adult cows (Internal report #16-06SRB; manuscript in preparation). One overall hypothesis could be that MCWF has the ability to attract neutrophils and increase their phagocytic activity along with an increase in an array of pro- and anti-inflammatory cytokines that could be beneficial in reducing clinical signs associated with BRDC. This correlates with the highest incidence of BRDC observed in calves upon entry into the feedlot during the first 14-21days.
The exact mode of action of MCWF in BRDC-associated morbidity remains unclear and additional in vitro and in vivo studies are required to better understand the positive effect observed in clinical settings following MCWF administration in feedlot animals. All previously generated data accompanied by our current findings from a pilot study on reduced morbidity and increased weight gain suggest that MCWF could be used as non-antibiotic prophylactic alternative for calves entering feed lots.

Figure 1 Incidence of morbidity (number of calves required the treatment during the study). Data were considered statistically significant if p<0.05.
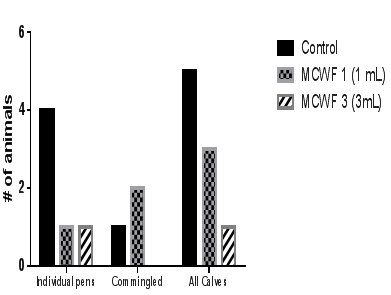
Figure 2 Incidence of mortality (number of calves required the treatment during the study). Data were considered statistically significant if p<0.05.
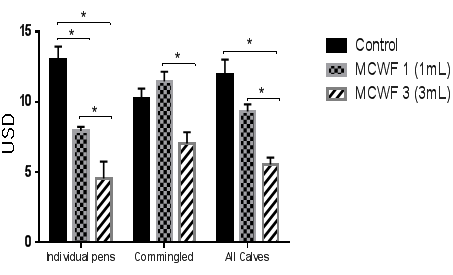
Figure 3 Average treatment cost (USD) per head. Data were considered statistically significant if p<0.05.
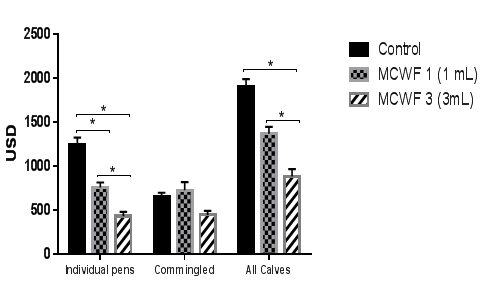
Figure 4 Total cost of treatment for each experimental group (USD). Data were considered statistically significant if p<0.05.
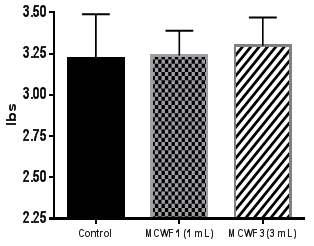
Figure 6 Average daily weight gain (ADG) per calf (lbs) during the 102days in feedlot (individual pens).
1mL MCWF (Amplimune™) |
3mL MCWF (Amplimune™) |
|
Drug Costs Saved (USD) |
$303.71 |
$ 750.71 ab |
Additional Weight Gain |
580 lb |
1,630 lb |
Fewer Deaths than Controls |
2 |
4 |
Fewer Treatments than Controls |
13 |
39 |
Table 2 Summary of beneficial MCWF between two MCWF groups compared to controls
Authors Nosky B, Alkemade S and Masic A were employees, or are still employed by Vetrepharm and NovaVive (companies which previously had or currently hold licensing rights for Amplimune™) at the time of study execution (Nosky, Alkemade) or manuscript preparation (Masic).

©2017 Nosky, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.